Balancing Redox Reactions Worksheet With Answers
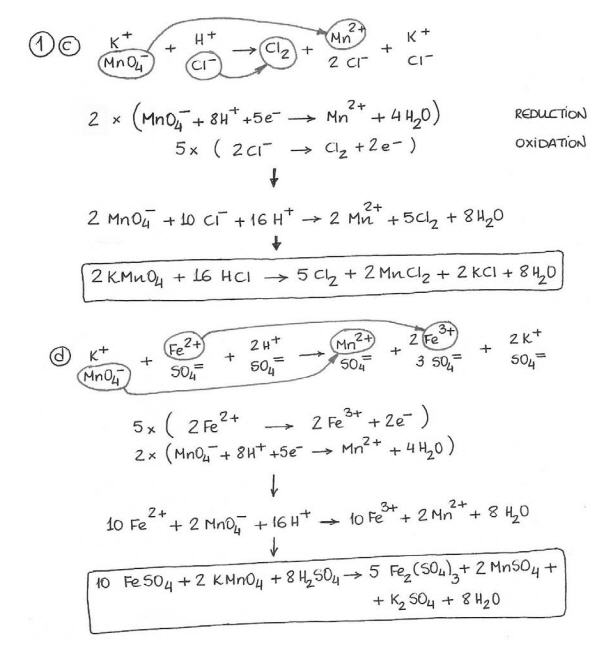
Balancing Redox Reactions Worksheet With Answers - Oxidation and reduction occur simultaneously in order to conserve charge. Web steps for balancing redox reactions. Web balancing redox reaction worksheet. Web download & view balancing redox reactions worksheets 1 & 2 (with answers) as pdf for free. Balance h by adding h +. Multiply by some integer to make electrons (lost) = electrons (gained) step 6: Ascorbic acid (c \text {}_ {6} 6 h \text {}_ {8} 8 o \text {}_ {6} 6) is a common antioxidant that protects our bodies against radicals. 2li + s li2s li. No s0, so, mno2 mn20 balance each redox reaction in acid solution using the half reaction method. Live worksheets > english > chemistry > redox > balancing redox reaction. Determine what is oxidized and what is reduced in each reaction. Ascorbic acid (c \text {}_ {6} 6 h \text {}_ {8} 8 o \text {}_ {6} 6) is a common antioxidant that protects our bodies against radicals. Web chemistry questions and answers; B2o3 +mg → mgo+mg3 b2 (neutral) b. Web balancing redox reaction worksheet. Identify the oxidizing and reducing agents. Assign oxidation numbers to all elements and identify those that are oxidized and reduced. If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). Identify the oxidizing agent and the reducing agent, also. Balance the charge or oxidation number with electrons. Multiply by some integer to make electrons (lost) = electrons (gained) step 6: Zn → zn 2+ reduction reaction: Web balance the following redox reactions: No s0, so, mno2 mn20 balance each redox reaction in acid solution using the half reaction method. In the first case you separate out the oxidation and reduction half reaction and in the second case,. Identify the oxidizing and reducing agents. Determine what is oxidized and what is reduced in each reaction. Assign oxidation numbers to all elements and identify those that are oxidized and reduced. Add half equations and cancel substances on both sides. They actually involve the same procedure. Web balance the following redox reactions: If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). Web balancing redox equations worksheet oxidation number method for balancing redox equations 1. Worksheet #5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. We can. Worksheet #5 balancing redox reactions in acid and basic solution balance each half reaction in basic solution. Balance the charge or oxidation number with electrons. Zn → zn 2+ reduction reaction: Web balancing redox reaction worksheet. The half reaction method and the change in oxidation method. Ascorbic acid (c \text {}_ {6} 6 h \text {}_ {8} 8 o \text {}_ {6} 6) is a common antioxidant that protects our bodies against radicals. Web download & view balancing redox reactions worksheets 1 & 2 (with answers) as pdf for free. If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine. Web balancing redox reactions: Worksheet on balancing redox equations two methods are often mentioned for balancing redox reactions: If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). Balance the charge or oxidation number with electrons. B2o3 +mg → mgo+mg3 b2 (neutral) b. Web balance the following redox reactions: No s0, so, mno2 mn20 balance each redox reaction in acid solution using the half reaction method. Balancing redox reactions worksheet balancing redox reactions using oxidation number method worksheet balance the following equations using the oxidation number method. Identify the oxidizing agent and the reducing agent, also. Web download & view balancing redox reactions. If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). 2 h + + vo 2 + → vo 2+ + h 2 o step 3: Balancing redox reactions worksheet balancing redox reactions using oxidation number method worksheet balance the following equations using the oxidation number method. Web. Web steps for balancing redox reactions. Worksheet on balancing redox equations two methods are often mentioned for balancing redox reactions: Identify the pair of elements undergoing oxidation and reduction by checking oxidation states; Oxidation and reduction occur simultaneously in order to conserve charge. Web balancing redox reaction worksheet. Web balance the following redox reactions: They actually involve the same procedure. Balance o by adding h 2 o. Web chemistry questions and answers; Identify the oxidizing and reducing agents. Add half equations and cancel substances on both sides. Balance h by adding h +. No s0, so, mno2 mn20 balance each redox reaction in acid solution using the half reaction method. If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). 2li + s li2s li. Vo 2 + → vo 2 + step 2: Web balancing redox reactions: 2 h + + vo 2 + → vo 2+ + h 2 o step 3: Identify the oxidizing agent and the reducing agent, also. Assign oxidation numbers to all elements and identify those that are oxidized and reduced. Worksheet on balancing redox equations two methods are often mentioned for balancing redox reactions: No s0, so, mno2 mn20 balance each redox reaction in acid solution using the half reaction method. Web balancing redox reactions: Oxidation and reduction occur simultaneously in order to conserve charge. Balancing redox reactions worksheet balancing redox reactions using oxidation number method worksheet balance the following equations using the oxidation number method. The half reaction method and the change in oxidation method. Identify the pair of elements undergoing oxidation and reduction by checking oxidation states; They actually involve the same procedure. Web steps for balancing redox reactions. Ascorbic acid (c \text {}_ {6} 6 h \text {}_ {8} 8 o \text {}_ {6} 6) is a common antioxidant that protects our bodies against radicals. B2o3 +mg → mgo+mg3 b2 (neutral) b. 2 h + + vo 2 + → vo 2+ + h 2 o step 3: Web balancing redox equations worksheet oxidation number method for balancing redox equations 1. Vo 2 + → vo 2 + step 2: If only one element is both oxidized and reduced (disproportionation), write it down twice (then recombine it after the equation is balanced). We can “see” these changes if we assign oxidation numbers to the reactants and.Balancing Redox Reactions Worksheets 1 & 2 (With Answers)
Solved Worksheet Balancing Redox Equations (Numbers to the
Redox Reactions Worksheet With Answers Printable Worksheets and
Balancing+redox+reactions+worksheet+answers
Redox Reactions Worksheet Answer Key kidsworksheetfun
Balancing Redox Reactions Worksheet_key
Balancing Redox Reactions Worksheet With Answers worksheet
Balancing Redox Reactions Worksheet properinspire
35 Balancing Redox Equations Worksheet Answers Chemistry If8766
Balancing Redox Reactions Worksheet Answer Key Askworksheet
Live Worksheets > English > Chemistry > Redox > Balancing Redox Reaction.
Balance The Charge Or Oxidation Number With Electrons.
Zn → Zn 2+ Reduction Reaction:
Worksheet #5 Balancing Redox Reactions In Acid And Basic Solution Balance Each Half Reaction In Basic Solution.
Related Post: