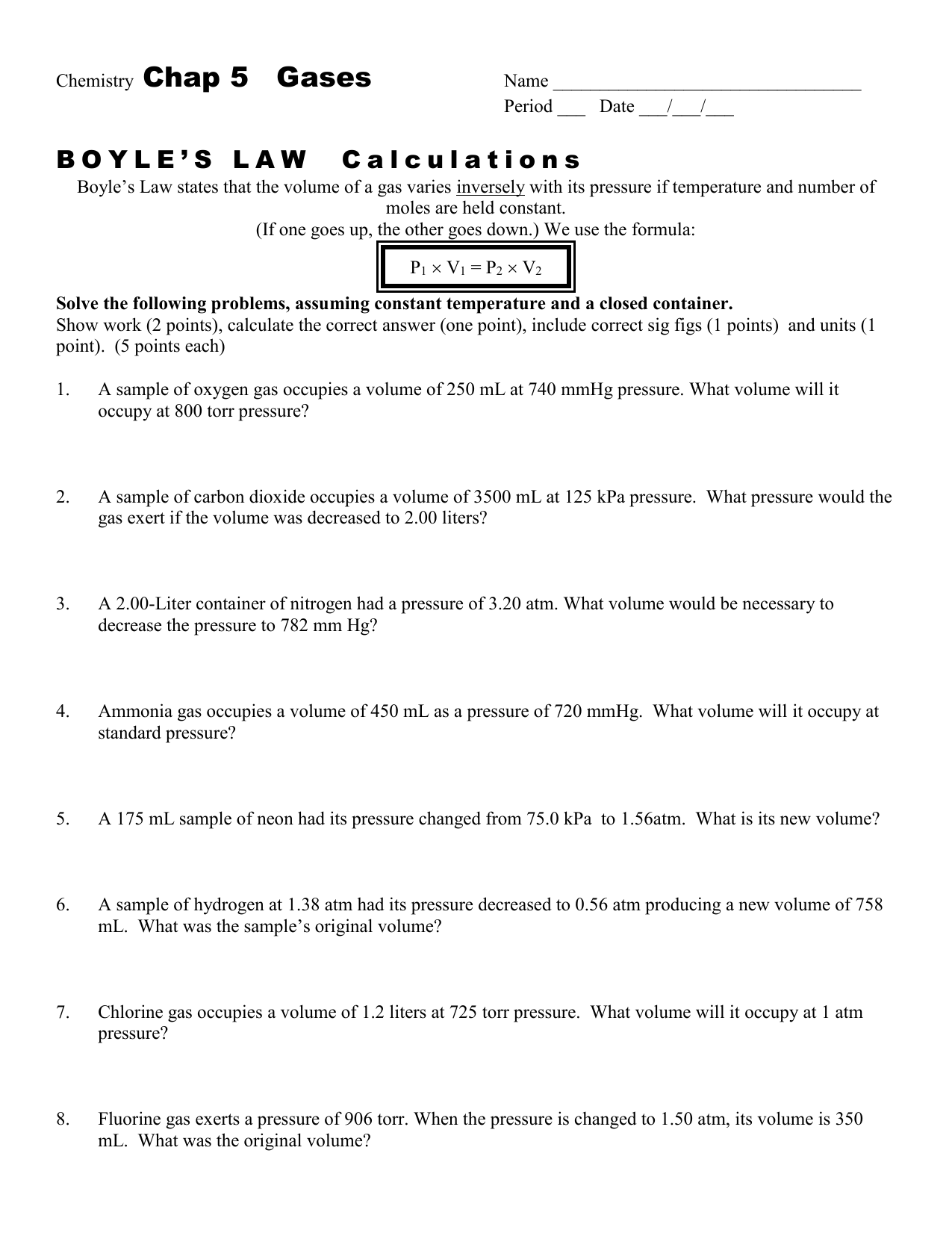
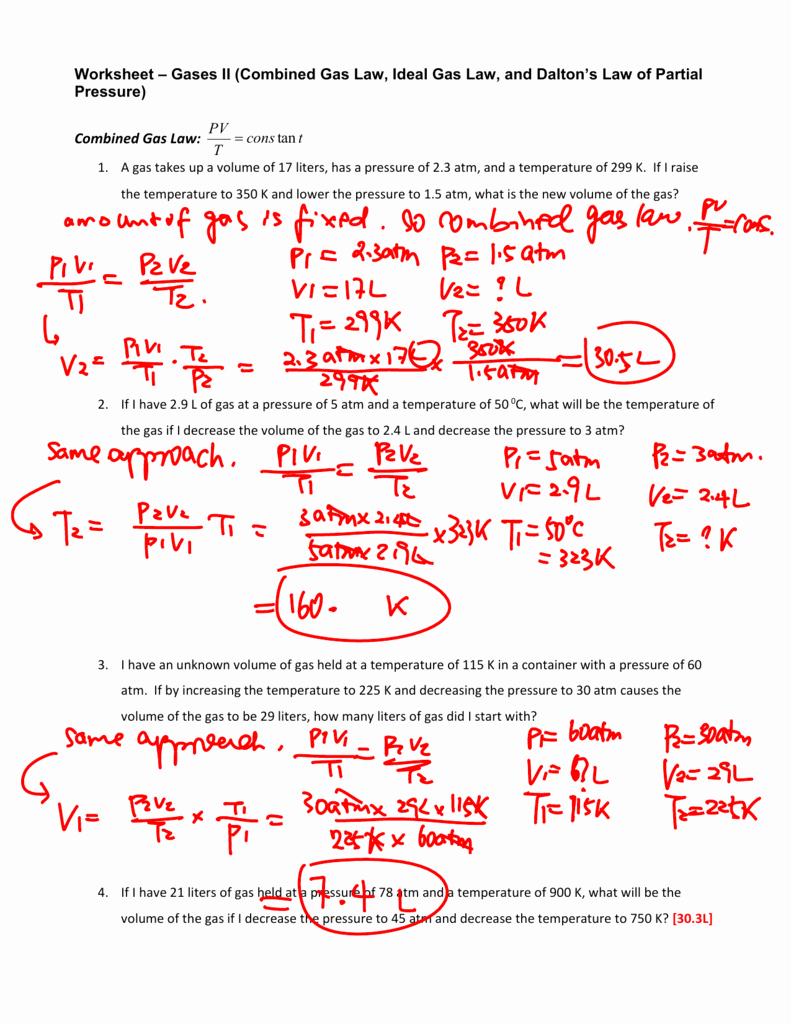
Boyle's Law Problems Worksheet Answers
Boyle's Law Problems Worksheet Answers - Web write a brief answer to the following questions. Sometimes a student will look at the temperature being cut in half and reason that the volume must also be cut in half. It is important that the two volumes (. A sample of oxygen gas. This problem is solved by inserting values into p1v1 = p2v2. 2.00 l of a gas is at 740.0 mmhg pressure. Web first of all, 2.20 l is the wrong answer. Solve the following problems showing all work. Boyle's law states that under conditions of constant temperature and quantity, there is an inverse relationship between the volume and pressure for an ideal. Web use boyle’s law to solve for the unknown pressure @$\\begin{align*}(p_2)\\end{align*}@$. A sample of oxygen gas. If a gas at 25 °c occupies 3 liters. This problem is solved by inserting values into p1v1 = p2v2. By completing this activity, 9thgrade physical science and chemistry students will learn to. The relationship between pressure and volume is only observed when the temperature. Web boyle’s law name_____ key _____ section_____ solve the following problems assuming constant temperature. This is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. By completing this activity, 9thgrade physical science and chemistry students will learn to. Web first of all, 2.20 l is the wrong answer. If 22.5 l of. This pdf worksheet gives students practice completing word problems in chemistry using these. 1 atm = 760.0 mm hg = 101.3 kpa. The relationship between pressure and volume is only observed when the temperature. It is important that the two volumes (. Web use boyle’s law to solve for the unknown pressure @$\\begin{align*}(p_2)\\end{align*}@$. If a gas at 25 °c occupies 3 liters. This is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. Boyle’s law depicts the relationship between the pressure, volume, and temperature of a. Some of the worksheets for this concept are 9 1516 more boyles law and charless law wkst, 9 1314.. The relationship between pressure and volume is only observed when the temperature. It is important that the two volumes (. A sample of oxygen gas. This is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. (740.0 mmhg) (2.00 l) =(760.0 mmhg) (x) example #1: 2.00 l of a gas is at 740.0 mmhg pressure. Web this worksheet will prepare students to solve simple problems over boyle’s law. (a), boyle’s law is valid only for ideal gases. The relationship between pressure and volume is only observed when the temperature. 12 = 3x x = 7x. 2.00 l of a gas is at 740.0 mmhg pressure. This pdf worksheet gives students practice completing word problems in chemistry using these. Boyle's law states that under conditions of constant temperature and quantity, there is an inverse relationship between the volume and pressure for an ideal. Boyle’s law depicts the relationship between the pressure, volume, and temperature of a.. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at. 1 atm = 760.0 mm hg = 101.3 kpa. (740.0 mmhg) (2.00 l) =(760.0 mmhg) (x) example #1: If you think it is necessary, you may use illustrations to support your answer. 1) solve the following equations for x, show your algebra steps! (a), boyle’s law is valid only for ideal gases. Web boyle’s law name_____ key _____ section_____ solve the following problems assuming constant temperature. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at. A sample of oxygen gas. If you think it is necessary, you may use illustrations to support your answer. 1) 1.00 l of a gas at standard. Some of the worksheets for this concept are 9 1516 more boyles law and charless law wkst, 9 1314. This pdf worksheet gives students practice completing word problems in chemistry using these. Sometimes a student will look at the temperature being cut in half and reason that the volume must also be. 1 atm = 760.0 mm hg = 101.3 kpa. The boyle’s law formula, which is succinctly expressed as p 1 v 1 = p 2 v 2, describes the relationship between the pressure and volume of a. Web write a brief answer to the following questions. Web this worksheet will prepare students to solve simple problems over boyle’s law. Some of the worksheets for this concept are 9 1516 more boyles law and charless law wkst, 9 1314. This pdf worksheet gives students practice completing word problems in chemistry using these. A sample of oxygen gas. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at. If a gas at 25 °c occupies 3 liters. Web boyle’s law name_____ key _____ section_____ solve the following problems assuming constant temperature. Choose an answer and hit 'next'. Boyle’s law depicts the relationship between the pressure, volume, and temperature of a. Boyle's law states that under conditions of constant temperature and quantity, there is an inverse relationship between the volume and pressure for an ideal. Web use boyle’s law to solve for the unknown pressure @$\\begin{align*}(p_2)\\end{align*}@$. Web boyle's law investigates the relationship between pressure and volume. Sometimes a student will look at the temperature being cut in half and reason that the volume must also be cut in half. This is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. Web first of all, 2.20 l is the wrong answer. Web boyle’s law formula. It is important that the two volumes (. (740.0 mmhg) (2.00 l) =(760.0 mmhg) (x) example #1: This is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. If a gas at 25 °c occupies 3 liters. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at. Web first of all, 2.20 l is the wrong answer. You must identify what variable that each number goes with. Boyle's law states that under conditions of constant temperature and quantity, there is an inverse relationship between the volume and pressure for an ideal. Some of the worksheets for this concept are 9 1516 more boyles law and charless law wkst, 9 1314. Boyle’s law depicts the relationship between the pressure, volume, and temperature of a. Web use boyle’s law to solve for the unknown pressure @$\\begin{align*}(p_2)\\end{align*}@$. The boyle’s law formula, which is succinctly expressed as p 1 v 1 = p 2 v 2, describes the relationship between the pressure and volume of a. 1) solve the following equations for x, show your algebra steps! Web boyle’s law formula. Solve the following problems showing all work. 1 atm = 760.0 mm hg = 101.3 kpa. Web this worksheet will prepare students to solve simple problems over boyle’s law.Boyle's Law Worksheet Answers
Boyles And Charles Law Worksheet Answers Boyle039S Law —
Boyle S Law Worksheet Answers Chapter 12 worksheet
Boyle S Law Worksheet Answers Chapter 12 worksheet
50 Boyle's Law Worksheet Answers Chessmuseum Template Library
Boyle039S Law And Charles Law Worksheet Answer Key —
Boyle27 S Law Problems Worksheet With Answers BETTER
Boyle's Law Worksheet Answers Chemistry If8766 Gosustainable
39 boyle's law worksheet key Worksheet For Fun
50 Boyle's Law Worksheet Answers Chessmuseum Template Library
12 = 3X X = 7X.
2.00 L Of A Gas Is At 740.0 Mmhg Pressure.
Web Write A Brief Answer To The Following Questions.
If You Think It Is Necessary, You May Use Illustrations To Support Your Answer.
Related Post: