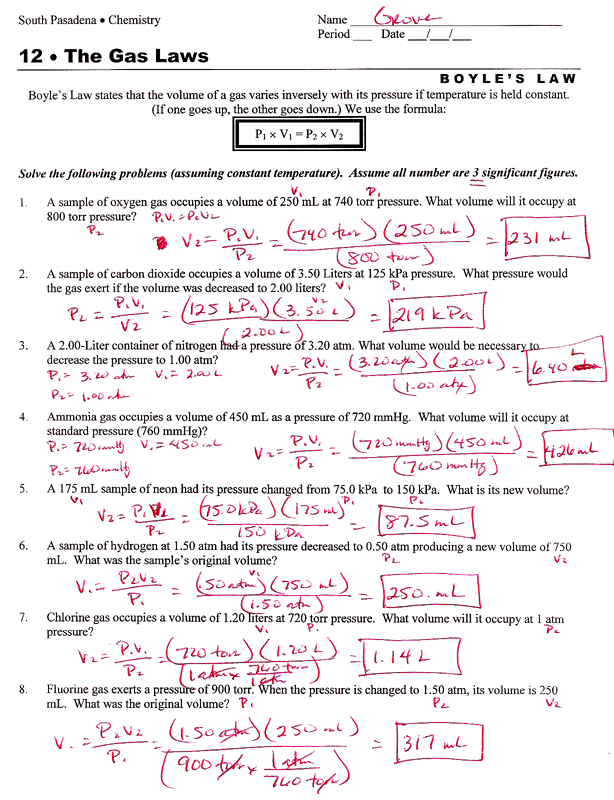
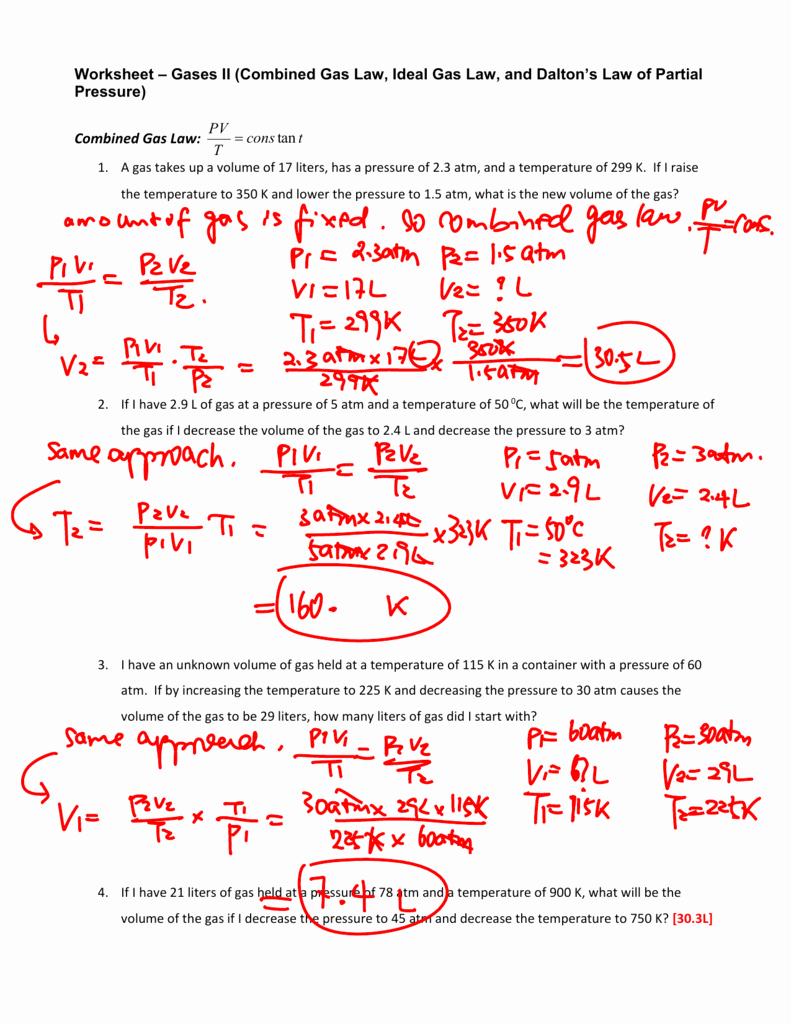
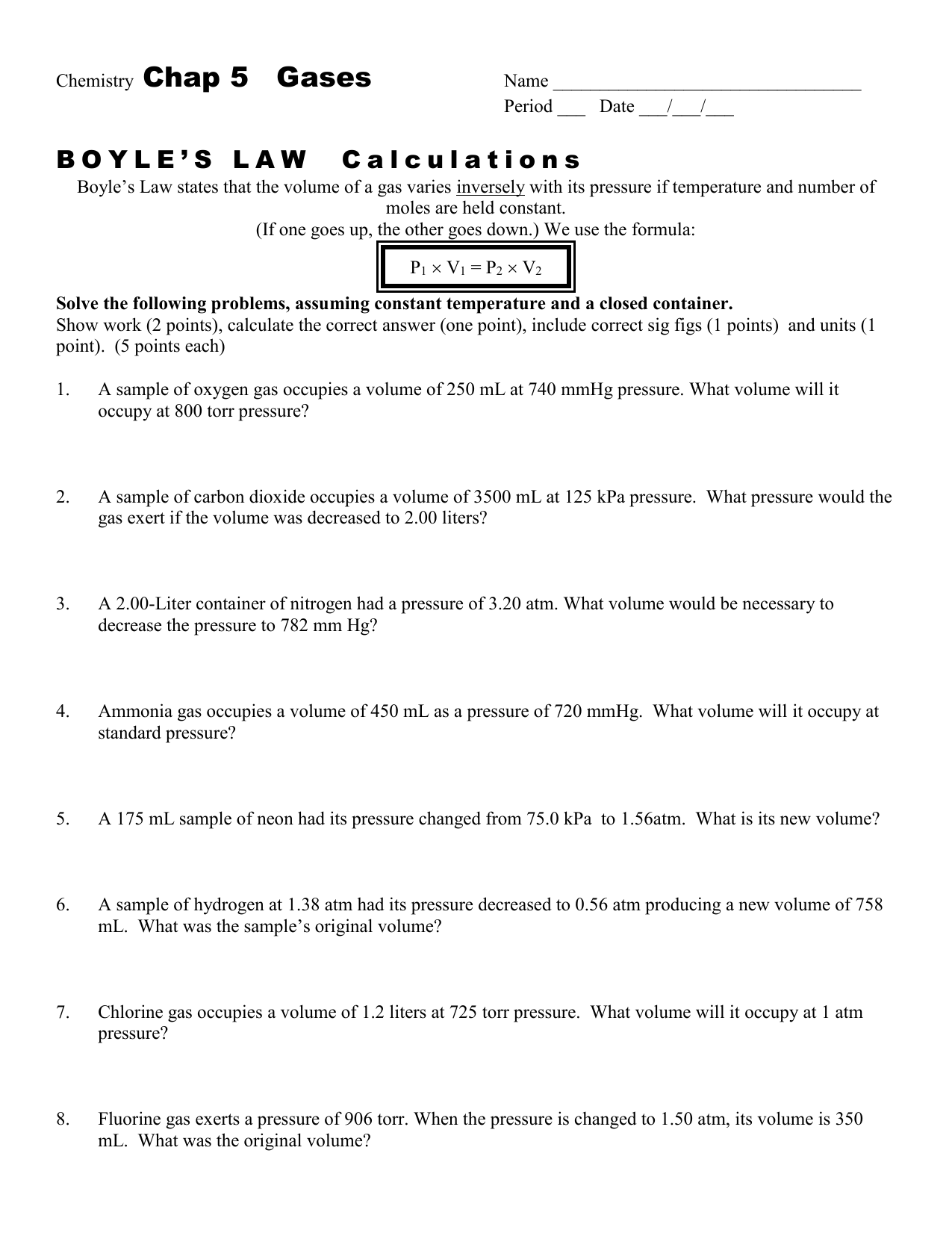
Boyles Law Worksheets
Boyles Law Worksheets - (if one goes up, the other. At constant temperature, pressure is inversely proportional to volume. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. If the pressure on a 1.00 l sample of gas is increased from 760. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? Boyle’s law is best of all because it presses gasses awfully small. Gas laws, boyles law, charles law. Web boyle’s regulation, charles legislation, homosexual lussac’s, mixed fuel law; 1 atm = 760.0 mm hg = 101.3 kpa. To verify boyle's law experimentally; Boyles law and charles law. The relationship between pressure and volume is only observed when the temperature and. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. Web boyle’s regulation, charles legislation, homosexual lussac’s, mixed fuel law; To verify boyle's law experimentally; In this worksheet, we will practice using the formula pv = constant (boyle’s law) to calculate the pressure or volume of a gas that is allowed to. Temperature and variety of moles of a fuel. Boyles law and charles law. Occupation (s) human rights lawyer and professor of law. The relationship between pressure and volume is only observed when the. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. To verify boyle's law experimentally; Web this is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. Mmhg to 2.00 atm at constant temperature, what will the volume of. Web zip a sandwich bag nearly closed. If the pressure on a 1.00 l sample of gas is increased from 760. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. With scaffolded questions, students will learn the relationship between gas pressure and. The equation of boyle's. In this worksheet, we will practice using the formula pv = constant (boyle’s law) to calculate the pressure or volume of a gas that is allowed to. Boyles law and charles law. Web this is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. (if one goes up, the other. Mmhg to. The equation of boyle's law; Occupation (s) human rights lawyer and professor of law. At constant temperature, pressure is inversely proportional to volume. Web this is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. Boyles law and charles law. Revisions may be submitted online by. State boyle's law in your own words. With scaffolded questions, students will learn the relationship between gas pressure and. Gas laws, boyles law, charles law. Insert a straw into the opening and blow through the straw to inflate the bag so that it is a little over half full of air. At constant temperature, pressure is inversely proportional to volume. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? In this worksheet, we will practice using the formula pv = constant (boyle’s law) to calculate the pressure or volume of a gas that is allowed to. Web boyle’s regulation, charles legislation, homosexual lussac’s, mixed. If the pressure on a 1.00 l sample of gas is increased from 760. Revisions may be submitted online by. Boyles law and charles law. Web this is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law. The relationship between pressure and volume is only observed when the temperature and. With scaffolded questions, students will learn the relationship between gas pressure and. The relationship between pressure and volume is only observed when the temperature and. Insert a straw into the opening and blow through the straw to inflate the bag so that it is a little over half full of air. (if one goes up, the other. If the pressure. The equation of boyle's law; Revisions may be submitted online by. Francis anthony boyle (born march 25, 1950) [1] is a human rights lawyer and professor of. With scaffolded questions, students will learn the relationship between gas pressure and. Boyles law and charles law. Boyle’s law states that the volume of a gas varies inversely with its pressure if temperature and number of moles is held constant. _____ _____ zip a sandwich. Web boyle’s regulation, charles legislation, homosexual lussac’s, mixed fuel law; Web zip a sandwich bag nearly closed. Temperature and variety of moles of a fuel. At constant temperature, pressure is inversely proportional to volume. In this worksheet, we will practice using the formula pv = constant (boyle’s law) to calculate the pressure or volume of a gas that is allowed to. The relationship between pressure and volume is only observed when the temperature and. State boyle's law in your own words. Insert a straw into the opening and blow through the straw to inflate the bag so that it is a little over half full of air. Occupation (s) human rights lawyer and professor of law. Charles regulation worksheet reply key. Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? 1 atm = 760.0 mm hg = 101.3 kpa. If the pressure on a 1.00 l sample of gas is increased from 760. Boyle’s law is best of all because it presses gasses awfully small. State boyle's law in your own words. Revisions may be submitted online by. Temperature and variety of moles of a fuel. Gas laws, boyles law, charles law. Boyles law and charles law. Occupation (s) human rights lawyer and professor of law. 1 atm = 760.0 mm hg = 101.3 kpa. If the pressure on a 1.00 l sample of gas is increased from 760. In this worksheet, we will practice using the formula pv = constant (boyle’s law) to calculate the pressure or volume of a gas that is allowed to. To verify boyle's law experimentally; Mmhg to 2.00 atm at constant temperature, what will the volume of the gas sample become? Web zip a sandwich bag nearly closed. _____ _____ zip a sandwich. The relationship between pressure and volume is only observed when the temperature and. Web this is expressed in the equation p1 × v1 = p2 × v2, which is known as boyle’s law.Boyle's Law Worksheet Name
12 Best Images of Boyles Law Worksheet Charles Law and Boyles Law
AP Chemistry THS January 2013
50 Boyle's Law Worksheet Answers Chessmuseum Template Library
boyle's law worksheet key
Boyles And Charles Law Worksheet Answers Boyle039S Law —
Boyle's Law Worksheet
Boyle's Law and Charles' Law Worksheet
50 Boyle's Law Worksheet Answers Chessmuseum Template Library
Chap 12 Boyle's Law Worksheet boyle's Law wksht 12
(If One Goes Up, The Other.
Boyle’s Law States That The Volume Of A Gas Varies Inversely With Its Pressure If Temperature And Number Of Moles Is Held Constant.
Insert A Straw Into The Opening And Blow Through The Straw To Inflate The Bag So That It Is A Little Over Half Full Of Air.
The Equation Of Boyle's Law;
Related Post: