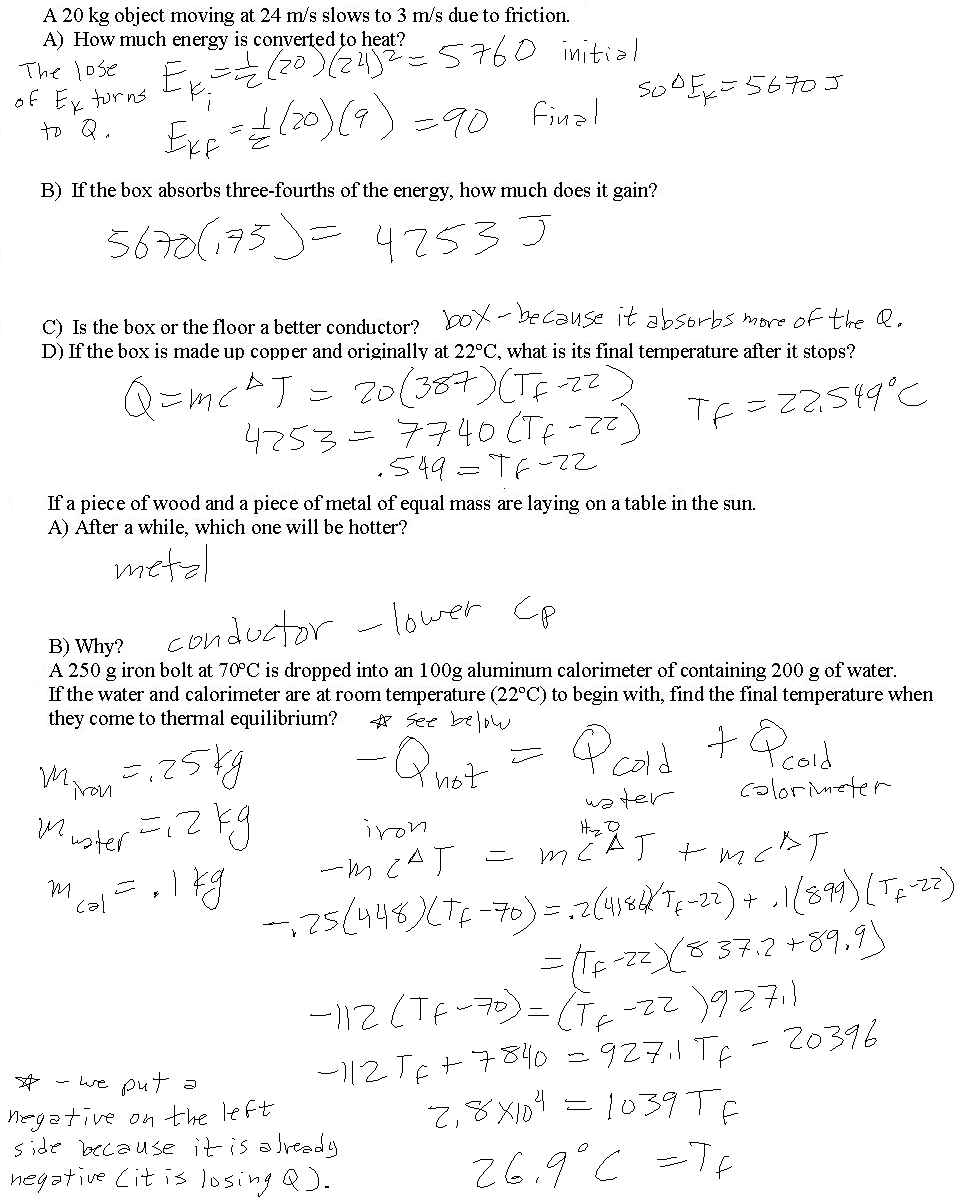Calorimetry Problems Worksheet Answers
Calorimetry Problems Worksheet Answers - The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature is closer to t_1 t 1 than to t_2 t 2. What are the two major ways in which the. Students will calculate for the variables temperature, mass, specific heat, etc. Choose an answer and hit 'next'. To do so, the heat is exchanged with a calibrated object (calorimeter). The specific heat of water = 4.18 j/goc. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry problems answers, calorimetry practice problems answers,. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. Web calorimetry problems worksheet 1. Choose an answer and hit 'next'. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? A reaction takes place in a calorimeter containing 400.0 g of water at an initial temperature. It’s about understanding the process of problem. Web calorimetry problems worksheet 1. The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature of the water was measured as 42.7 °c. What temperature change will 100.0 ml of water. To do so, the heat is exchanged with a calibrated object (calorimeter). Web worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry problems answers, calorimetry practice problems answers,. It’s about understanding the process of problem. The final temperature is closer to t_1 t 1 than to t_2 t 2. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c. The final temperature of the water was measured as 42.7 °c. Web specific heat and calorimetry (heat lost=heat gained) worksheet. The final temperature is closer to t_1 t 1 than to t_2 t 2. What temperature change will 100.0 ml of water. Students will calculate for the variables temperature, mass, specific heat, etc. Web calorimetry problems worksheet answers march 24, 2023 by tamble it isn’t just about getting the right answers; The final temperature of the water was measured as 42.7 °c. It’s about understanding the process of problem. The final temperature is closer to t_1 t 1 than to t_2 t 2. What are the two major ways in which the. The final temperature is closer to t_1 t 1 than to t_2 t 2. A reaction takes place in a calorimeter containing 400.0 g of water at an initial temperature. The final temperature is exactly. It’s about understanding the process of problem. To do so, the heat is exchanged with a calibrated object (calorimeter). Students will calculate for the variables temperature, mass, specific heat, etc. The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature is exactly. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. The specific heat of water = 4.18 j/goc. (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; It’s about understanding the process of problem. What temperature change will 100.0 ml of water. Web calorimetry problems worksheet 1. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature of the water was measured as 42.7 °c. A reaction takes place in a calorimeter containing 400.0 g of water at an initial temperature. Web specific heat and calorimetry (heat lost=heat gained) worksheet. To do so, the heat is exchanged with a calibrated. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? Web calorimetry problems worksheet 1. To do so, the heat is exchanged with a calibrated object (calorimeter). The final temperature is closer to t_1 t 1 than to t_2 t 2. What are the two major ways in which the. Students will calculate for the variables temperature, mass, specific heat, etc. Web specific heat and calorimetry (heat lost=heat gained) worksheet. A reaction takes place in a calorimeter containing 400.0 g of water at an initial temperature. Web worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry problems answers, calorimetry practice problems answers,. (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius? Web calorimetry is used to measure amounts of heat transferred to or from a substance. Web calorimetry problems worksheet answers march 24, 2023 by tamble it isn’t just about getting the right answers; What are the two major ways in which the. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? It’s about understanding the process of problem. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. To do so, the heat is exchanged with a calibrated object (calorimeter). The specific heat of water = 4.18 j/goc. Choose an answer and hit 'next'. What temperature change will 100.0 ml of water. The final temperature is exactly. The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature of the water was measured as 42.7 °c. The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature is closer to t_1 t 1 than to t_2 t 2. To do so, the heat is exchanged with a calibrated object (calorimeter). Web calorimetry problems worksheet answers march 24, 2023 by tamble it isn’t just about getting the right answers; What are the two major ways in which the. Web worksheets are calorimetry work w 337, ii calorimetry work, chemistry work heat and calorimetry problems answers, calorimetry practice problems answers,. The final temperature is closer to t_1 t 1 than to t_2 t 2. The final temperature is exactly. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web calorimetry is used to measure amounts of heat transferred to or from a substance. Web specific heat and calorimetry (heat lost=heat gained) worksheet. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. It’s about understanding the process of problem. Choose an answer and hit 'next'. What temperature change will 100.0 ml of water. Web calorimetry problems worksheet 1. Web how much heat is released when 275 grams of water cools from 85.2 degrees celsius to 28.4 degrees celsius?30 Calorimetry Worksheet Answer Key Education Template
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Mr Murray's Website Two Dim Motion
30 Calorimetry Worksheet Answer Key Education Template
Calorimetry Worksheet Answers Chapter 5 Thermochemistry / Back to
Calorimetry Worksheet Answer Key
Calorimetry Problems Worksheet Specific Heat And Calorimetry Packet
30 Calorimetry Worksheet Answer Key Education Template
Calorimetry Worksheet Answers
Calorimetry Practice Worksheet Answers
The Specific Heat Of Water = 4.18 J/Goc.
A Reaction Takes Place In A Calorimeter Containing 400.0 G Of Water At An Initial Temperature.
(Heat Capacity Is The Amount Of Energy To Increase The Temperature Of An Object By 1 Oc;
Students Will Calculate For The Variables Temperature, Mass, Specific Heat, Etc.
Related Post: