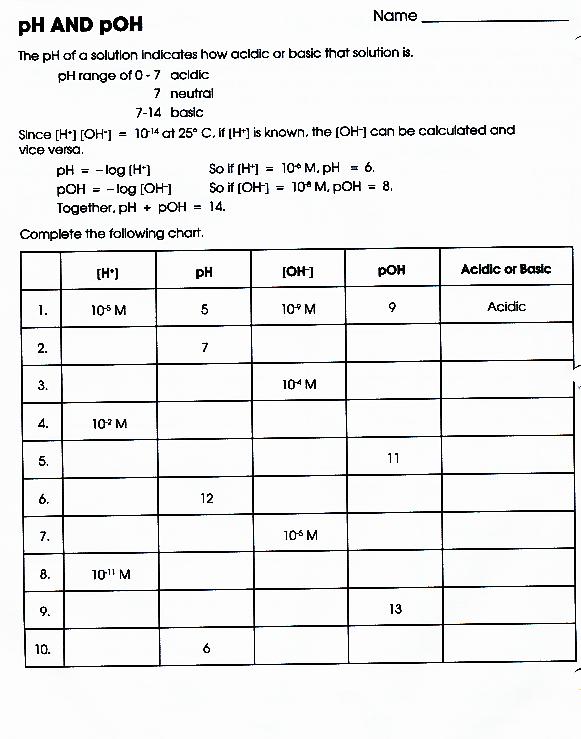
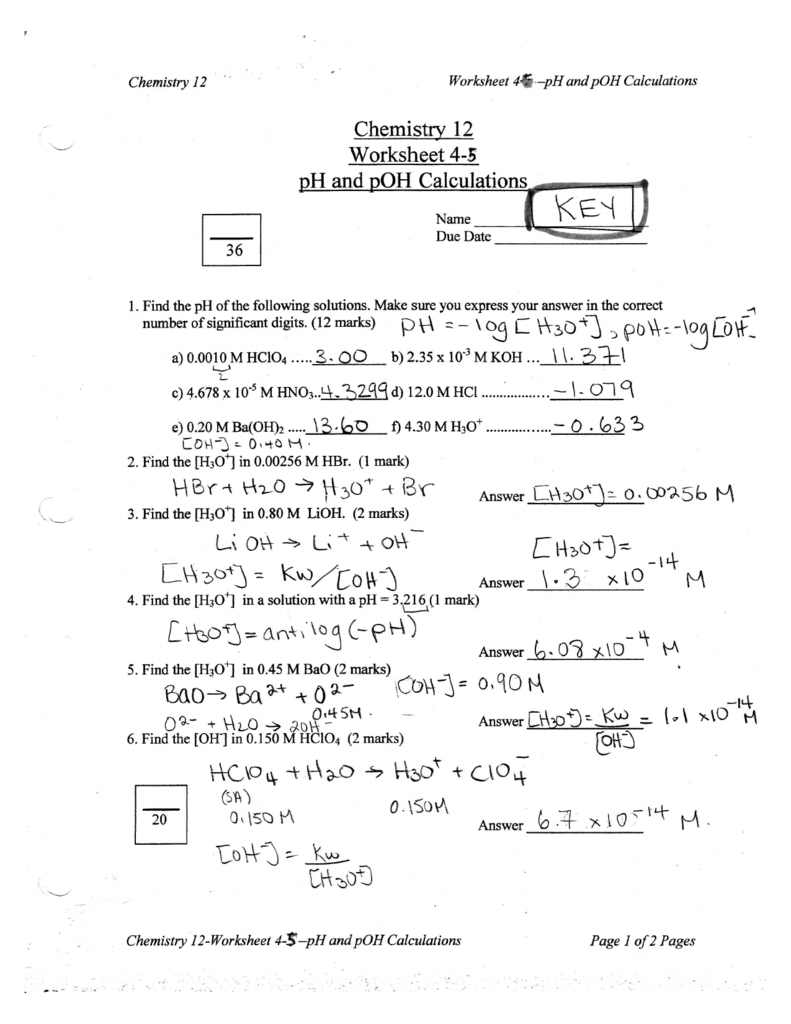
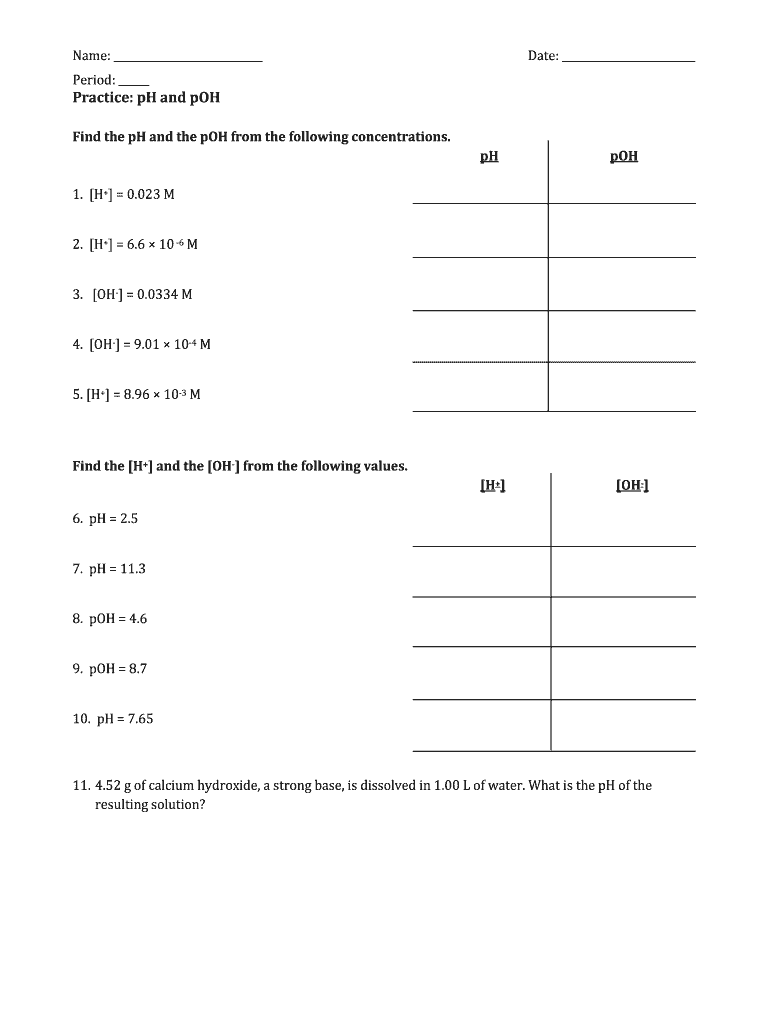
Chemistry Ph And Poh Worksheet Answer Key
Chemistry Ph And Poh Worksheet Answer Key - Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Find the [hsc ] in a solution in which the poh = 4.50. The ph and poh of a neutral solution. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web up to 6% cash back the sum of ph and poh is always 14. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. Web 14.00 = ph + poh. Web crash course chemistry #30 (ph and poh) worksheet. Web phil and poh are define as the adverse log of hydrogen ion concentration and chemical concentration, respectively. Fill in the missing information in the table below. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? The. Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Ph gives the acidity of a substance. Poh are related to ph and cans be well charging. Web crash course chemistry #30 (ph and poh) worksheet. Web we can now calculate the poh, and then ph using the ph + ph = 14 relationship: The ph and poh of a neutral solution. The ph is given by the formula: This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. Fill in the missing information in the table below. Web calculate the ph by rearranging and plugging in the poh: As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Web we can now calculate the poh, and then ph using the ph + ph = 14 relationship: Find the [hsc ] in a solution in which the poh = 4.50. Web poh =. Ph gives the acidity of a substance. Fill in the missing information in the table below. Uopnps puy noá dn0jb jnoá ssnosya Yellow blue red ph, poh, k a & pk a worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or. Web 100tps.10] yo hd suopnps jo 01 pasgv.1. Yellow blue red ph, poh, k a & pk a worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or. Web calculate the ph by rearranging and plugging in the poh: Find the [hsc ] in a solution in which the poh = 4.50. As was shown in example 14.1,. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. The ph is given by the formula: Poh are related to. Poh are related to ph and cans be well charging. Ph gives the acidity of a substance. Web up to 6% cash back the sum of ph and poh is always 14. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. Web if. Web crash course chemistry #30 (ph and poh) worksheet. Web course handouts » chemistry » unit. Fill in the missing information in the table below. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m. Poh are related to ph and cans be well charging. Ph gives the acidity of a substance. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution?. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic solutions exhibit ph less than 6.31 and. Web up to 6% cash back the sum of ph and poh is always 14. Ph gives the acidity of a substance. Uopnps puy noá dn0jb jnoá ssnosya Yellow blue red ph, poh, k a & pk a worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or. Poh are related to ph and cans be well charging. Web calculate the ph by rearranging and plugging in the poh: Web course handouts » chemistry » unit. Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web phil and poh are define as the adverse log of hydrogen ion concentration and chemical concentration, respectively. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Find the [hsc ] in a solution in which the poh = 4.50. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. Web 14.00 = ph + poh. Web ph and poh. Web we can now calculate the poh, and then ph using the ph + ph = 14 relationship: Fill in the missing information in the table below. A 0 m solution of magnesium hydroxide. Web crash course chemistry #30 (ph and poh) worksheet. Web poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. The ph is the measure of the power of hydrogen in a substance. A 0 m solution of magnesium hydroxide. Fill in the missing information in the table below. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Web we can now calculate the poh, and then ph using the ph + ph = 14 relationship: Web crash course chemistry #30 (ph and poh) worksheet. The ph and poh of a neutral solution. Web ph and poh. This is because the product of proton concentration and hydroxide concentration must always equal the equilibrium constant. Ph gives the acidity of a substance. Poh are related to ph and cans be well charging. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web up to 6% cash back the sum of ph and poh is always 14. Web calculate the ph by rearranging and plugging in the poh:Ph and Poh Worksheet Worksheet Template Tips
Ph And Poh Worksheet Answers
Chemistry 12 worksheet 4 3 ph and poh calculations
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
Ph And Poh Worksheet Answers
Ph And Poh Calculations Worksheet Answer Key CALCULATOR CGW
31 Ph Worksheet Answer Key Education Template
Ph And Poh Worksheet Answers
30 Ph and Poh Worksheet Education Template
Chemistry 12 Worksheet 43 Ph And Poh Calculations Answer Key → Waltery
Find The [Hsc ] In A Solution In Which The Poh = 4.50.
At This Temperature, Then, Neutral Solutions Exhibit Ph = Poh = 6.31, Acidic Solutions Exhibit Ph Less Than 6.31 And.
Uopnps Puy Noá Dn0Jb Jnoá Ssnosya
Web Phil And Poh Are Define As The Adverse Log Of Hydrogen Ion Concentration And Chemical Concentration, Respectively.
Related Post: