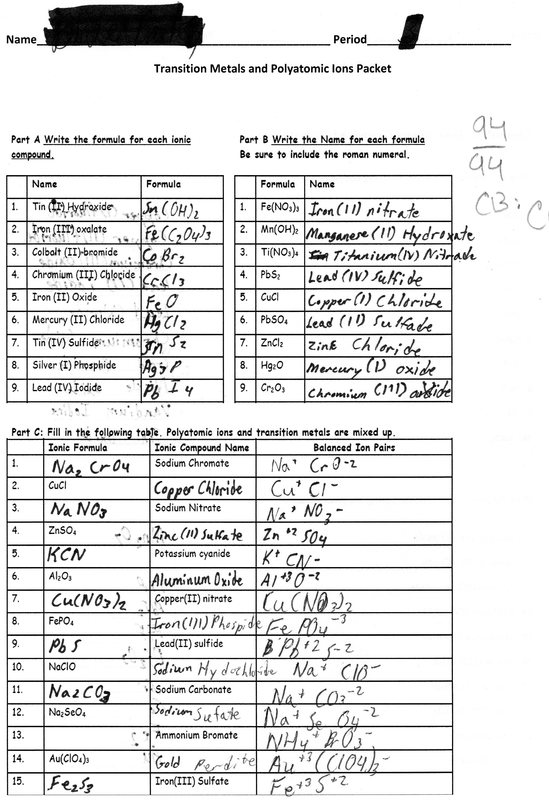
Formulas And Nomenclature Binary Ionic Transition Metals Worksheet
Formulas And Nomenclature Binary Ionic Transition Metals Worksheet - Types of compounds types of ions: Ionic compounds do not exist as molecules. In the solid state, ionic compounds are in a crystal. Metals combine with nonmetals to give ionic compounds. The positive ion (called a cation) is named first and the negative ion. Web 1.1 chemistry in context. Web practice naming binary ionic compounds, including those with transition metals, with this 32 problem worksheet. Web “ide” form the name of an anion. Nibr 2 nickel(ii) bromide 2. Web formulas and nomenclature binary ionic transition metals worksheet name the following compounds. First, students learn about ionic bonding and what makes a compound ionic, including electronegativity differences. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. Metals combine with nonmetals to give ionic compounds. In the solid state, ionic compounds are in a crystal. The positive ion (called a cation) is named first and the negative. Web “ide” form the name of an anion. Web the nomenclature for binary ionic compounds simply entails naming the ions according to the following rules: Nibr 2 nickel(ii) bromide 2. When naming binary ionic compounds, name the cation first (specifying the charge, if. 1.3 physical and chemical properties. Web students will learn how to assign roman numerals to transition metals to indicate their charge, and how to use anion charges to figure out the oxidation number of a transition. Perfect for classwork, homework, extra practice, or as examples. When naming binary ionic compounds, name the cation first (specifying the charge, if. Web this unit covers all aspects of. When two different elements combine, they form a binary compound. When naming binary ionic compounds, name the cation first (specifying the charge, if. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. The charge of the metal ion must be written in the name of the compound with. Web “ide” form the name of. Web this printable was uploaded at march 05, 2023 by tamble in ionic. Web write the correct names and formulas for binary ionic compounds. Web students will learn how to assign roman numerals to transition metals to indicate their charge, and how to use anion charges to figure out the oxidation number of a transition. Fe 2 o 3 iron(iii). The charge of the metal ion must be written in the name of the compound with. Web the nomenclature for binary ionic compounds simply entails naming the ions according to the following rules: Web formulas and nomenclature binary ionic transition metals worksheet name the following compounds. Web write the correct names and formulas for binary ionic compounds. Web this printable. First, students learn about ionic bonding and what makes a compound ionic, including electronegativity differences. When two different elements combine, they form a binary compound. Fe 2 o 3 iron(iii) oxide. Web practice naming binary ionic compounds, including those with transition metals, with this 32 problem worksheet. Nibr 2 nickel(ii) bromide 2. Ionic compounds do not exist as molecules. Fe 2 o 3 iron(iii) oxide. The positive ion (called a cation) is named first and the negative ion. The charge of the metal ion must be written in the name of the compound with. Web practice naming binary ionic compounds, including those with transition metals, with this 32 problem worksheet. Nibr 2 nickel(ii) bromide 2. 1.5 measurement uncertainty, accuracy, and precision. In the solid state, ionic compounds are in a crystal. Web write the correct names and formulas for binary ionic compounds. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. Web “ide” form the name of an anion. Web students will learn how to assign roman numerals to transition metals to indicate their charge, and how to use anion charges to figure out the oxidation number of a transition. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. Web this printable was uploaded at. The positive ion (called a cation) is named first and the negative ion. When two different elements combine, they form a binary compound. In the solid state, ionic compounds are in a crystal. Web this unit covers all aspects of ionic compounds. 1.3 physical and chemical properties. First, students learn about ionic bonding and what makes a compound ionic, including electronegativity differences. We have seen some ionic binary. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. Metals combine with nonmetals to give ionic compounds. The charge of the metal ion must be written in the name of the compound with. Nibr 2 nickel(ii) bromide 2. Naming ionic compounds with transition metals requires the use of a roman numeral. Web 1.1 chemistry in context. Web students will learn how to assign roman numerals to transition metals to indicate their charge, and how to use anion charges to figure out the oxidation number of a transition. 1.2 phases and classification of matter. Perfect for classwork, homework, extra practice, or as examples. Web practice naming binary ionic compounds, including those with transition metals, with this 32 problem worksheet. 1.5 measurement uncertainty, accuracy, and precision. Ionic compounds do not exist as molecules. Web this printable was uploaded at march 05, 2023 by tamble in ionic. Web practice naming binary ionic compounds, including those with transition metals, with this 32 problem worksheet. Web “ide” form the name of an anion. Web formulas and nomenclature binary ionic transition metals worksheet name the following compounds. We have seen some ionic binary. Web the nomenclature for binary ionic compounds simply entails naming the ions according to the following rules: In the solid state, ionic compounds are in a crystal. Web this printable was uploaded at march 05, 2023 by tamble in ionic. Fe 2 o 3 iron(iii) oxide. 1.3 physical and chemical properties. 1.5 measurement uncertainty, accuracy, and precision. 1.2 phases and classification of matter. Ionic formulas (binary, polyatomic, transition metals) write the formula for each of the following compounds. Perfect for classwork, homework, extra practice, or as examples. Naming ionic compounds with transition metals requires the use of a roman numeral. Ionic compounds do not exist as molecules. Types of compounds types of ions:Formulas And Nomenclature Binary Ionic Transition Metals Worksheet
Formulas And Nomenclature Binary Ionic Compounds Worksheet Answers — db
31 Simple Binary Ionic Compounds Worksheet Education Template
27 hiragana charts stroke order practice mnemonics and adobe acrobat
√ 20 Naming Binary Ionic Compounds Worksheet Simple Template Design
42 formulas and nomenclature binary ionic transition metals worksheet
Name writing compounds with transition metals
Chemistry
Binary Ionic Compounds Worksheet —
30 Naming Binary Ionic Compounds Worksheet Education Template
When Naming Binary Ionic Compounds, Name The Cation First (Specifying The Charge, If.
Metals Combine With Nonmetals To Give Ionic Compounds.
Web Students Will Learn How To Assign Roman Numerals To Transition Metals To Indicate Their Charge, And How To Use Anion Charges To Figure Out The Oxidation Number Of A Transition.
When Two Different Elements Combine, They Form A Binary Compound.
Related Post: