Gas Laws Worksheet Answers
Gas Laws Worksheet Answers - Web use your knowledge of the ideal and combined gas laws to solve the following problems. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Each of these laws can be derived. Web this puzzle is a great review of gas laws, unit conversions, and general terms related to gases.there is both a full color and a black and white option along with answer keys. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If the gases in the can reach a pressure of 90 lbs/in2, the can will. The following practice problems are to master to topics on the ideal gas laws: An activity worksheet that reviews the ideal gas law equation incorporating the four individual gas laws (boyle's law, gay lucas's law,. Web gases and gas laws worksheet. Click the card to flip 👆. Atm = 760 mm hg = 101 kpa= 760.0 torr. An activity worksheet that reviews the ideal gas law equation incorporating the four individual gas laws (boyle's law, gay lucas's law,. Web gases and gas laws worksheet. Each of these laws can be derived. N = pv = (2.8 atm)(98 l) = 11. If the gases in the can reach a pressure of 90 lbs/in2, the can will. Web use your knowledge of the ideal and combined gas laws to solve the following problems. Web this puzzle is a great review of gas laws, unit conversions, and general terms related to gases.there is both. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Convert this to mmhg and psi. Each of these laws can be derived. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. 1 atm = 760.0 mm hg = 101.3 kpa. Click the card to flip 👆. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Each of these laws can be derived. The average atmospheric pressure on mount everest is 0.311 atm. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Atm = 760 mm hg = 101 kpa= 760.0 torr. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. Click the card to flip. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. Each of these laws can be derived. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. The following practice problems are to master to topics on the ideal gas laws: An activity worksheet that reviews the ideal. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Atm = 760 mm hg = 101 kpa= 760.0 torr. Web gases and gas laws worksheet. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. Click the card to flip 👆. Click the card to flip 👆. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. The average atmospheric pressure on mount everest is 0.311 atm. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Web. An activity worksheet that reviews the ideal gas law equation incorporating the four individual gas laws (boyle's law, gay lucas's law,. Web gases and gas laws worksheet. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. N = pv = (2.8 atm)(98 l) = 11. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm. 1 atm = 760.0 mm hg = 101.3 kpa. Click the card to flip 👆. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Atm = 760 mm hg = 101 kpa= 760.0 torr. The average atmospheric pressure on mount everest is 0.311 atm. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. Web use your knowledge of the ideal and combined gas laws to solve the following problems. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If the gases in the can reach a pressure of 90 lbs/in2, the can will. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. The following practice problems are to master to topics on the ideal gas laws: N = pv = (2.8 atm)(98 l) = 11. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Atm = 760 mm hg = 101 kpa= 760.0 torr. The average atmospheric pressure on mount everest is 0.311 atm. Click the card to flip 👆. Each of these laws can be derived. Web this puzzle is a great review of gas laws, unit conversions, and general terms related to gases.there is both a full color and a black and white option along with answer keys. 1 atm = 760.0 mm hg = 101.3 kpa. Web gases and gas laws worksheet. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. An activity worksheet that reviews the ideal gas law equation incorporating the four individual gas laws (boyle's law, gay lucas's law,. Convert this to mmhg and psi. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant. If the gases in the can reach a pressure of 90 lbs/in2, the can will. Web gases and gas laws worksheet. An activity worksheet that reviews the ideal gas law equation incorporating the four individual gas laws (boyle's law, gay lucas's law,. N = pv = (2.8 atm)(98 l) = 11. 1 atm = 760.0 mm hg = 101.3 kpa. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. Web this puzzle is a great review of gas laws, unit conversions, and general terms related to gases.there is both a full color and a black and white option along with answer keys. Web use your knowledge of the ideal and combined gas laws to solve the following problems. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. The following practice problems are to master to topics on the ideal gas laws: Convert this to mmhg and psi.Worksheet On Ideal Gas Equation Ideal Gas Law Worksheet Answer Key
Combined Gas Law Worksheet Answers
combined gas law practice worksheet
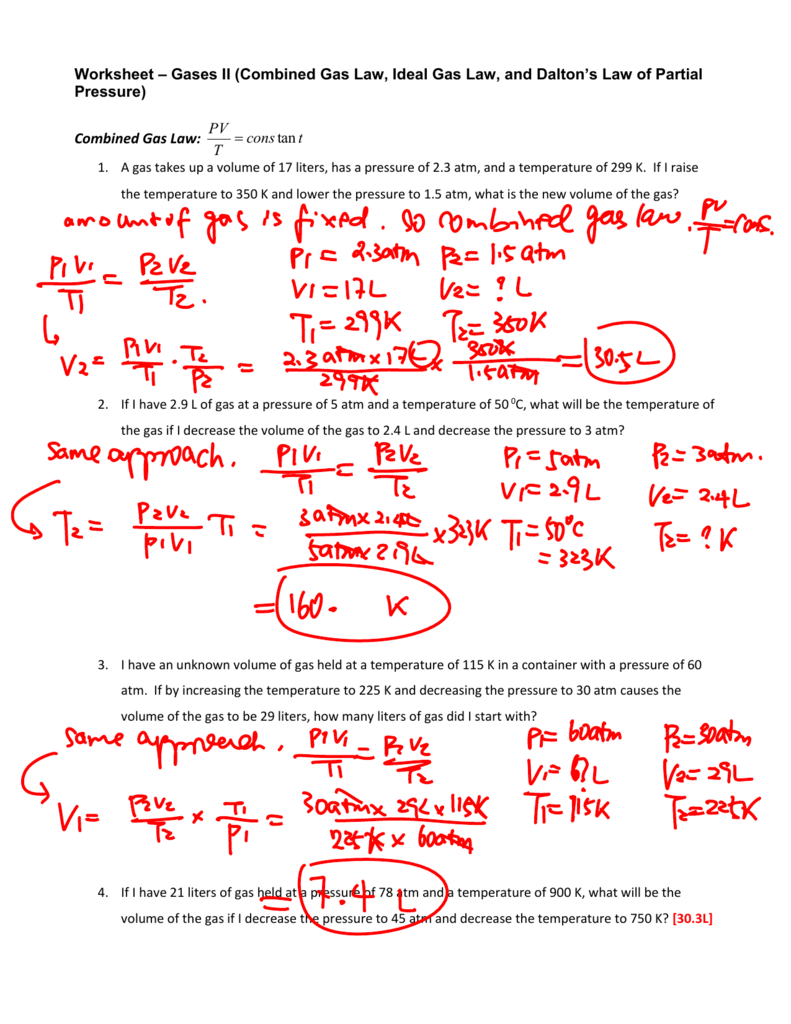
Worksheet Gas Laws II Answers
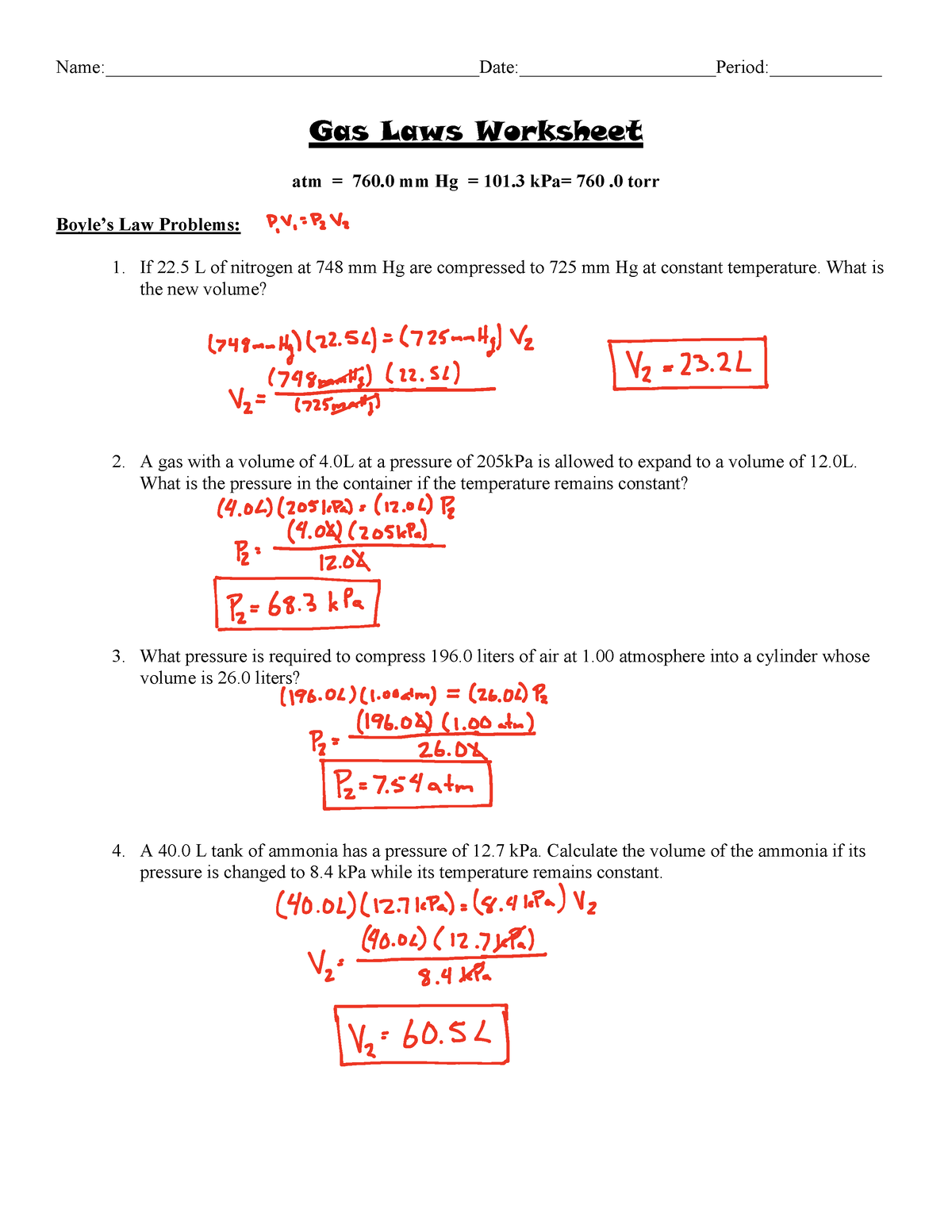
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg
Combined Gas Law Worksheet Answers
Gas Variables Worksheet Answers Word Worksheet
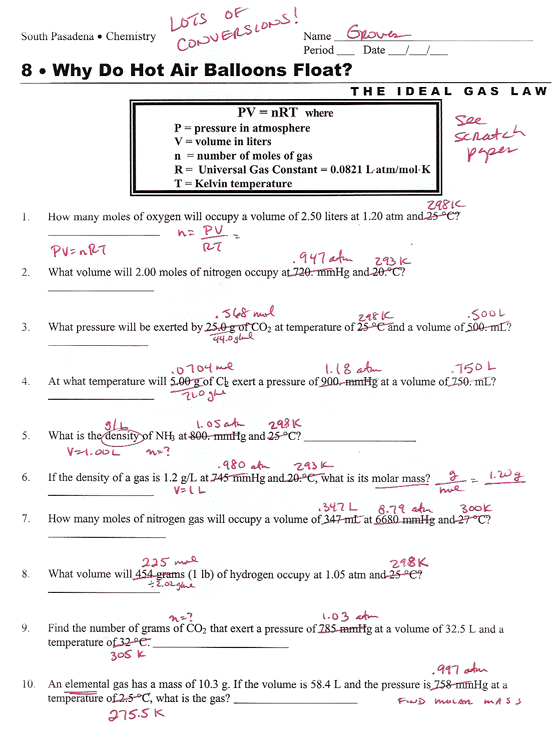
Ideal Gas Law Worksheet 144 Answer Key Greenus
17 Mixed Gas Laws Worksheet Answers /
15 Charles Law And Boyle's Law Worksheet /
Click The Card To Flip 👆.
Atm = 760 Mm Hg = 101 Kpa= 760.0 Torr.
The Average Atmospheric Pressure On Mount Everest Is 0.311 Atm.
Each Of These Laws Can Be Derived.
Related Post: