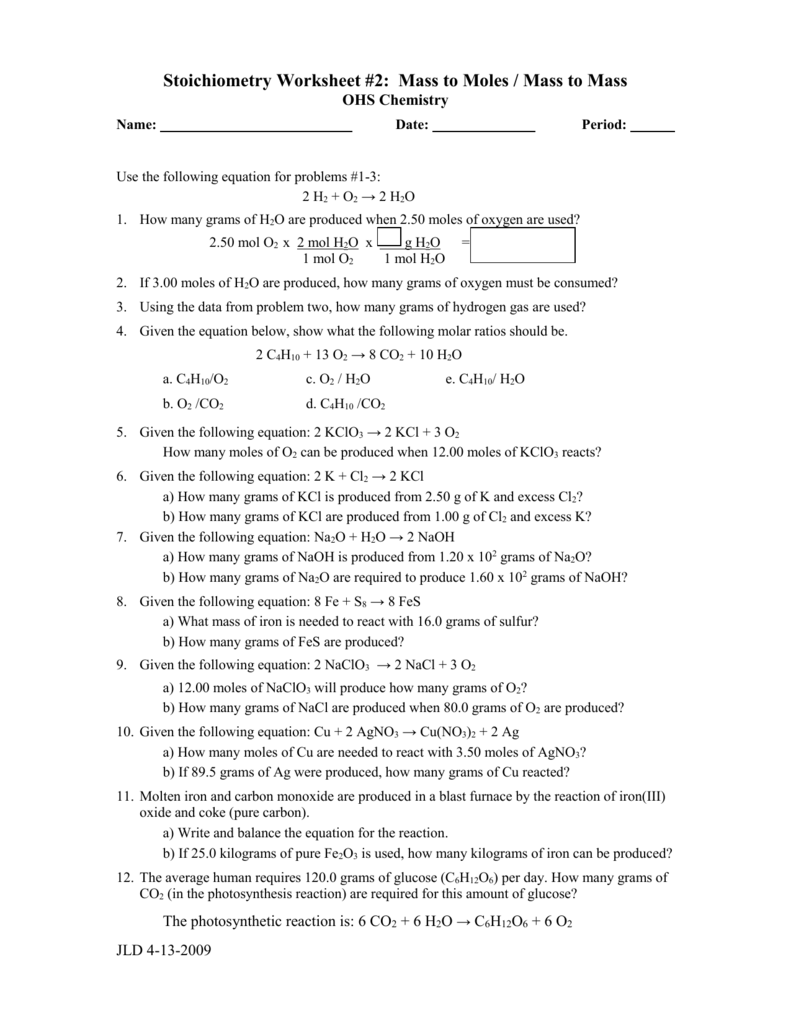
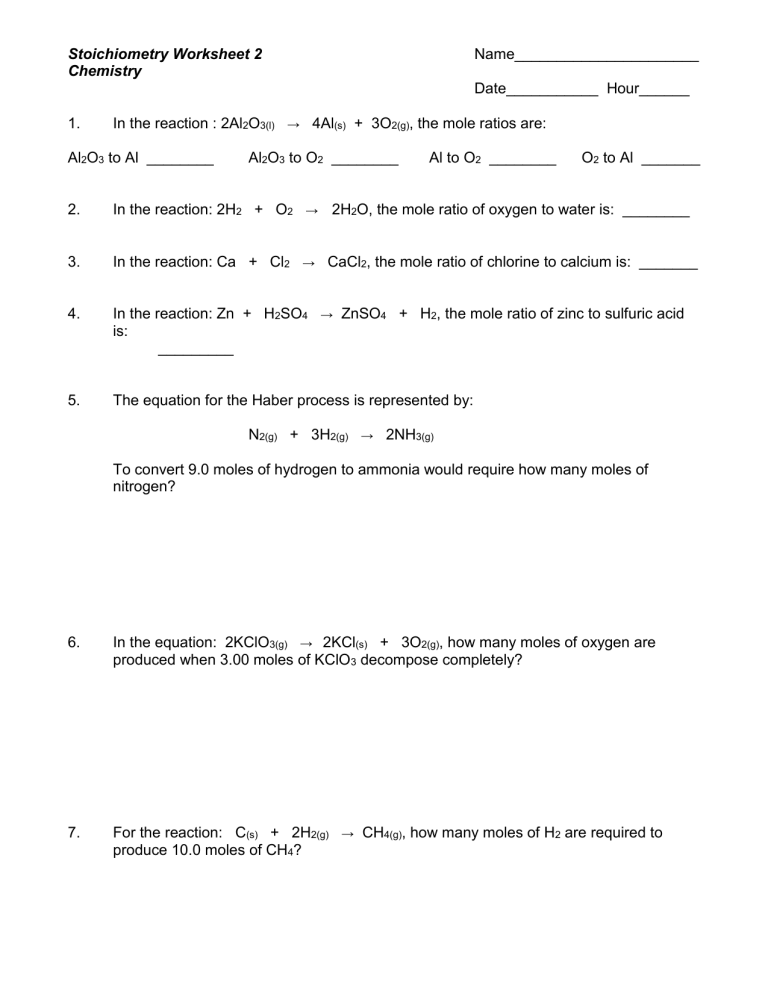
Gram To Gram Stoichiometry Worksheet
Gram To Gram Stoichiometry Worksheet - Converting between grams and moles (stoichiometry basics) id: Calculate the number of moles of naoh that are needed to react with 500.0 g of. Show your work, including proper units, to earn full credit. H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. Web stoichiometry calculation practice worksheet. Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. H2so4according to the following equation:. Gram to gram worksheet.pdf author: Solve each of the following problems. Web stoichiometry calculation practice worksheet. H2so4according to the following equation:. Gram to gram worksheet.pdf author: Calculate the number of moles of naoh that are needed to react with 500 g of h 2 so 4 according to the following equation: Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. Convert the following number of moles. It walks through each conversion with more detail. Convert the following number of moles of chemical into its corresponding mass in grams. Web often it is helpful to assume a sample size of exactly 100 grams; Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. How many grams of aluminum. Web stoichiometry calculation practice worksheet. Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the. Web stoichiometry calculation practice worksheet. Web up to 24% cash back a. Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. Calculate the number of moles of naoh that are needed to react with 500 g of h 2 so 4. Web up to 24% cash back a. How many grams of aluminum oxide will be formed from 17 grams of aluminum reacting? From 1.00 g of cl2 and excess k? Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. H2so4according to the following equation:. Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. H2so4according to the following equation:. Gram to gram worksheet.pdf author: H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. Web up to 24% cash back a. From 1.00 g of cl2 and excess k? How many grams of oxygen are needed to react with 12.8 grams of. Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. Calculate the volume of carbon dioxide gas produced at stp if 100.0 grams of. H2so4according to the following equation:. Convert the following number of moles of chemical into its corresponding mass in grams. H2so4according to the following equation:. How many grams of oxygen are needed to react with 12.8 grams of. Web stoichiometry calculation practice worksheet. Gram to gram worksheet.pdf author: Calculate the volume of carbon dioxide gas produced at stp if 100.0 grams of. H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. Web how many grams of kcl is produced from 2.50 g of k and excess cl2. This is the third of four stoichiometry worksheets. Web. Show your work, including proper units, to earn full credit. Calculate the volume of carbon dioxide gas produced at stp if 100.0 grams of. Converting between grams and moles (stoichiometry basics) id: Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. How many grams of aluminum oxide will be formed. Gram to gram worksheet.pdf author: Web stoichiometry calculation practice worksheet. Calculate the volume of carbon dioxide gas produced at stp if 100.0 grams of. How many grams of aluminum oxide will be formed from 17 grams of aluminum reacting? Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. Web often it is helpful to assume a sample size of exactly 100 grams; It walks through each conversion with more detail. How many grams of oxygen are needed to react with 12.8 grams of. If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the. Web up to 24% cash back a. Then the given percentages are numerically equal to the number of grams of each element. Web how many grams of kcl is produced from 2.50 g of k and excess cl2. This is the third of four stoichiometry worksheets. Calculate the number of moles of naoh that are needed to react with 500.0 g of. Web (i) grams of hi m= n •m = 0.14 mol • 127.91 g/mol = 17.91 g of hi are needed as a reactant (ii) grams of cui m= n •m = 0.07 mol • 190.45 g/mol = 13.33 g of cui are created as. Converting between grams and moles (stoichiometry basics) id: Show your work, including proper units, to earn full credit. Web this worksheet contains over 5 high quality gram to gram stoichiometry problems, complete with full, detailed answer keys. Calculate the mass of ethanol produced if 500.0 grams of glucose reacts completely. Web stoichiometry calculation practice worksheet. Calculate the volume of carbon dioxide gas produced at stp if 100.0 grams of. If it takes 27.4 ml of 0.768 m n a o h to titrate 16.7 ml of h 2 s o 4, what is the. Show your work, including proper units, to earn full credit. Web worksheet for basic stoichiometry. Calculate the number of moles of naoh that are needed to react with 500 g of h 2 so 4 according to the following equation: Convert the following number of moles of chemical into its corresponding mass in grams. H 2 s o 4 + n a o h → n a 2 s o 4 + h 2 o. Web here’s a video on mole to mole, mole to grams, and grams to grams conversion. Then the given percentages are numerically equal to the number of grams of each element. It walks through each conversion with more detail. From 1.00 g of cl2 and excess k? Web (i) grams of hi m= n •m = 0.14 mol • 127.91 g/mol = 17.91 g of hi are needed as a reactant (ii) grams of cui m= n •m = 0.07 mol • 190.45 g/mol = 13.33 g of cui are created as. H2so4according to the following equation:. Web how many grams of kcl is produced from 2.50 g of k and excess cl2. Web often it is helpful to assume a sample size of exactly 100 grams; How many grams of aluminum oxide will be formed from 17 grams of aluminum reacting?Grams to Grams Stoichiometry Step by Step YouTube
gram to gram stoichiometry worksheet
Solved Stoichiometry Worksheet 1 GramMole Conversions
gram to gram stoichiometry worksheet
Grams to Moles Worksheet
Stoichiometry Worksheet 3 Gram To Gram Calculations Answers
Chemistry Stoichiometry Worksheet
️Gram To Gram Stoichiometry Worksheet Free Download Qstion.co
grams to grams stoichiometry worksheet
Stoichiometry Worksheet 2 (1)
Converting Between Grams And Moles (Stoichiometry Basics) Id:
Web Stoichiometry Calculation Practice Worksheet.
Web Up To 24% Cash Back A.
Calculate The Mass Of Ethanol Produced If 500.0 Grams Of Glucose Reacts Completely.
Related Post: