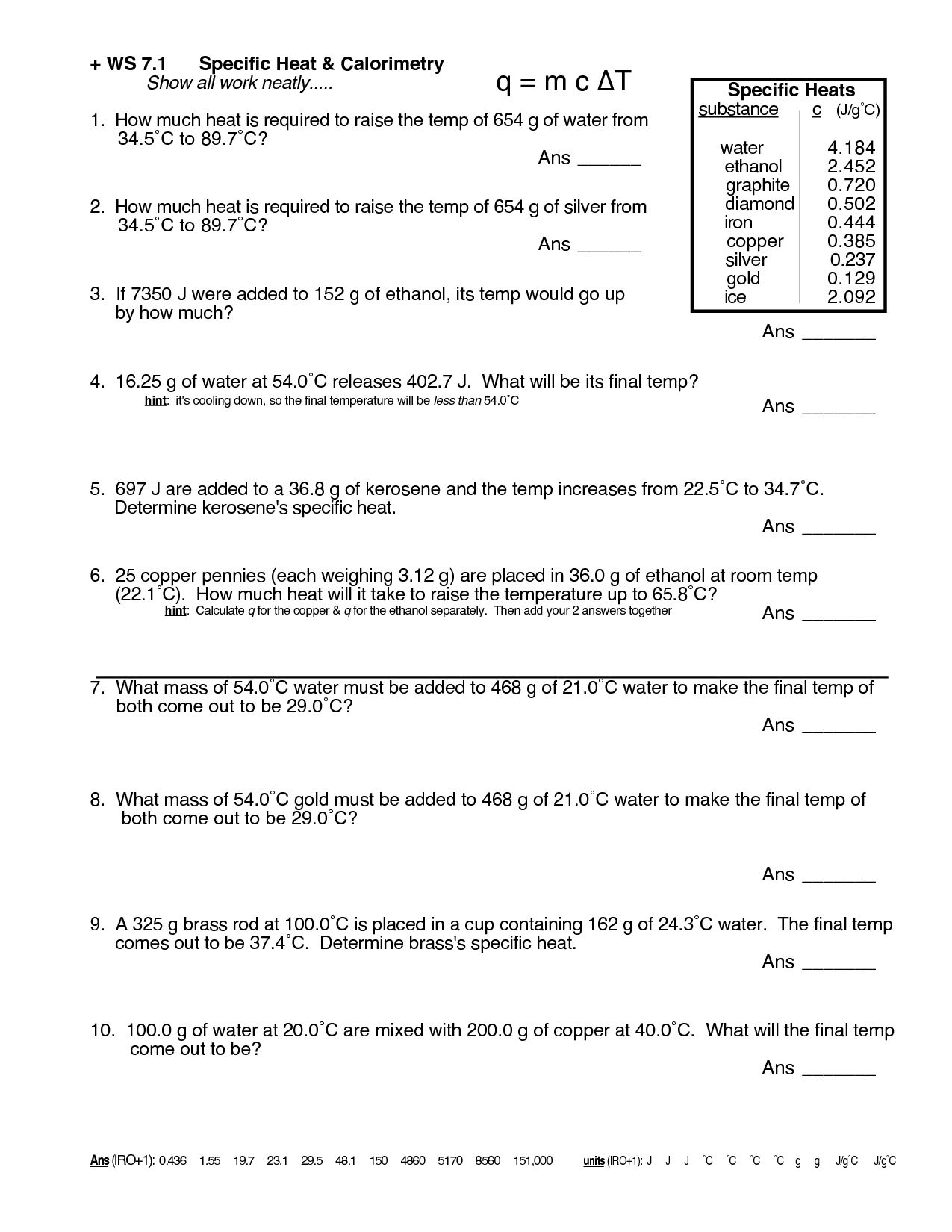
Heat Calculations Worksheet Answers
Heat Calculations Worksheet Answers - Use q = (m)(cp))(δt) to solve the following problems. For q= m c δ t : Topics you will need to know for the quiz include temperature and. A piece of copper with a mass of 218 g has heat. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Web the specific heat calculations worksheet consists of two pages:page 1: Web particular heat worksheet with answers pdf worksheet. Kj/mol solve by writing formation. Web calorimetry worksheet answers pdf curve heating pdffiller form. Show all work and units. Use q = (m)(cp))(δt) to solve the following problems. Energy is stored is energy in motion. Ag 2 s(s) + 2hcl(g) 2agcl(s) + h 2 s(g) h = ? Understand the relationships between heat, work, internal energy, and enthalpy. Show all work and units. A piece of copper with a mass of 218 g has heat. For q= m c δ t : Web the specific heat calculations worksheet consists of two pages:page 1: For q= m c δ t :. Show all work and units. Heat is not the same as temperature, yet they. Web the specific heat calculations worksheet consists of two pages:page 1: Specific heat calculations, comparing specific heats of materials, interpreting heating and cooling graphs of materials, making predictions, calculating rate. 25.0 g of mercury is heated from 25°c to 155°c, and absorbs 455 joules of heat in the process. (the specific. How many joules of heat are. Topics you will need to know for the quiz include temperature and. Write the formula for specific heat calculations. Web know the first law of thermodynamics. The specific heat c is a property of the substance; For q= m c δ t : Ag 2 s(s) + 2hcl(g) 2agcl(s) + h 2 s(g) h = ? (the specific heat of iron is 0.45\; 25.0 g of mercury is heated from 25°c to 155°c, and absorbs 455 joules of heat in the process. A piece of copper with a mass of 218 g has heat. Web the specific heat calculations worksheet consists of two pages:page 1: How many joules of heat are. (the specific heat of iron is 0.45\; Web which of the following is true of the final temperature of the system when thermal equilibrium is reached? What is the difference in temperature and heat? Its si unit is j/(kg ⋅ ⋅. Web this quiz and worksheet will help you quickly gauge your knowledge of heat energy and how it is calculated. Web calorimetry worksheet answers pdf curve heating pdffiller form. Use q = (m)(cp))(δt) to solve the following problems. Show all work and units. Web specific heat calculations worksheet. (the specific heat of iron is 0.45\; Show all work and units. Kj/mol solve by writing formation. Identify each variable and their units. A piece of copper with a mass of 218 g has heat. Web calorimetry worksheet answers pdf curve heating pdffiller form. Web this quiz and worksheet will help you quickly gauge your knowledge of heat energy and how it is calculated. Specific heat calculations worksheet briefencounters.ca. Web heat energy needed to cause this rise in temperature. 25.0 g of mercury is heated from 25°c to 155°c, and absorbs 455 joules of heat in the process. Write the formula for specific heat calculations. Web which of the following is true of the final temperature of the system when thermal equilibrium is reached? Energy is stored is energy in motion. Web the specific heat is the amount of. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. Kj/mol solve by writing formation. Web specific heat calculations worksheet. Web specific heat and heat capacity worksheet directions: Understand the concepts of heat. For q= m c δ t : Ag 2 s(s) + 2hcl(g) 2agcl(s) + h 2 s(g) h = ? Energy is stored is energy in motion. What is the difference in temperature and heat? Web the specific heat calculations worksheet consists of two pages:page 1: Specific heat calculations, comparing specific heats of materials, interpreting heating and cooling graphs of materials, making predictions, calculating rate. The specific heat c is a property of the substance; The answer key’s a separate google sheet. Web which of the following is true of the final temperature of the system when thermal equilibrium is reached? A piece of copper with a mass of 218 g has heat. Identify each variable and their units. For q= m c δ t : Write the formula for specific heat calculations. Understand the relationships between heat, work, internal energy, and enthalpy. Show all work and units. Calculate the h value heat for the following reaction: Heat is not the same as temperature, yet they. Web the specific heat calculations worksheet consists of two pages:page 1: Its si unit is j/(kg ⋅ ⋅. Specific heat calculations worksheet briefencounters.ca. For q= m c δ t : The specific heat c is a property of the substance; Web specific heat and heat capacity worksheet directions: Specific heat calculations, comparing specific heats of materials, interpreting heating and cooling graphs of materials, making predictions, calculating rate. Understand the relationships between heat, work, internal energy, and enthalpy. Identify each variables by name & the units associated with it. Web this quiz and worksheet will help you quickly gauge your knowledge of heat energy and how it is calculated. Web the specific heat is the amount of heat necessary to change the temperature of 1.00 kg of mass by 1.00 ºc. The answer key’s a separate google sheet. Understand the concepts of heat. Kj/mol solve by writing formation.Specific Heat Worksheet Answer Key —
Heat Calculations Worksheet Answers —
Specific Heat Answers 2013 Heat Capacity
Specific Heat And Heat Capacity Worksheet Answers Live Worksheet Online
Specific Heat Calculations Worksheet —
High School Physics Worksheets with Answers Pdf Briefencounters
Heat Calculations Worksheet Answers worksheet
Specific Heat Worksheet With Answers Pdf worksheet
Heat and Heat Calculations Worksheet
Specific Heat Worksheet Answers
Ag 2 S(S) + 2Hcl(G) 2Agcl(S) + H 2 S(G) H = ?
Use Q = (M)(Cp))(Δt) To Solve The Following Problems.
Web Specific Heat Calculations Worksheet.
Web Know The First Law Of Thermodynamics.
Related Post: