Ka And Kb Calculations Worksheet
Ka And Kb Calculations Worksheet - 9 h 7 o 4. [h+] = (k a[ha]) 1/2 31. Name:_period:_ ka and kb calculations Web vsb blogs | vsb blog site A worksheet or teacher lead problems & examples of calculating ka values of acids or. [h+] = k a d. The effect of dilution on weak acids and bases is different: Web this interactive quiz and printable worksheet will help you understand the concepts and definitions associated with the ka and kb of acids and. The first disinfectant used by joseph lister was called carbolic acid. Web therefore, kw = ka*kb equilibrium and acid/base chemistry 1. 9 h 7 o 4. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. The weak base ethylamine (c2h5nh2) has a kb of 6.4 x 104 a) write the equilibrium equation for the ionization of. Calculate the [h3o+] in a 0.45 m solution. So, kb = kw / ka 2. Web this interactive quiz and printable worksheet will help you understand the concepts and definitions associated with the ka and kb of acids and. The ph of a household ammonia. Web calculate the kb for the reaction. The weak base ethylamine (c2h5nh2) has a kb of 6.4 x 104 a) write the equilibrium. The first disinfectant used by joseph lister was called carbolic acid. Calculate the [h3o+] in a 0.45 m solution of hydrogen sulphide (h2s). If a solution of weak acid,. Web the acid dissociation constant is k a. Strong acids and strong bases completely dissociate i.e. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. [h+] = k a d. The ph of a household ammonia. How do you calculate kb? [h +] = k ak b[ha] b. Calculate the ph in a 0.60 m. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. Find the ph of a solution prepared by dissolving 1.61g of nac3h3o2 in enough water to make 835ml of solution. The weak base ethylamine (c2h5nh2) has a. A worksheet or teacher lead problems & examples of calculating ka values of acids or. [h+] = k a d. Name:_period:_ ka and kb calculations Strong acids and strong bases completely dissociate i.e. Web calculate the kb for the reaction. Web vsb blogs | vsb blog site [h+] = (k a[ha]) 1/2 31. Calculate the ph of a. Web therefore, kw = ka*kb equilibrium and acid/base chemistry 1. So, kb = kw / ka 2. Web therefore, kw = ka*kb equilibrium and acid/base chemistry 1. Calculate the ph in a 0.60 m. Calculate the [h3o+] in a 0.45 m solution of hydrogen sulphide (h2s). How do you calculate kb? Find the ph of a solution prepared by dissolving 1.61g of nac3h3o2 in enough water to make 835ml of solution. Name:_period:_ ka and kb calculations How do you calculate kb? Calculate the ph in a 0.60 m. Web this interactive quiz and printable worksheet will help you understand the concepts and definitions associated with the ka and kb of acids and. [h+] = k a d. Please show all work and answer clearly on your own paper. [h+] = k a d. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. Acetic acid is a weak monoprotic acid. Calculate the [h3o+] in a 0.45 m solution of hydrogen sulphide. Web the acid dissociation constant is k a. 1.a student has an acid with the molecular formula of hc. A worksheet or teacher lead problems & examples of calculating ka values of acids or. How do you calculate kb? The ph of a household ammonia. [h+] = k a d. So, kb = kw / ka 2. It's concentration is 0.20m and it's equilibrium concentration of h+ ions is 0.0019m. In short, diluting a strong acid or base has a direct logarithmic effect on ph. Strong acids and strong bases completely dissociate i.e. Web therefore, kw = ka*kb equilibrium and acid/base chemistry 1. The first disinfectant used by joseph lister was called carbolic acid. Find the ph of a solution prepared by dissolving 1.61g of nac3h3o2 in enough water to make 835ml of solution. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. Web vsb blogs | vsb blog site 9 h 7 o 4. [h+] = k a[ha] e. Name:_period:_ ka and kb calculations [h +] = k ak b[ha] b. Acetic acid is a weak monoprotic acid. [h+] = k a[ha] e. Acetic acid is a weak monoprotic acid. 9 h 7 o 4. You can calculate k by dividing kw by the value of ka, which will be equal to the kb constant or kw = kb x ka. How do you calculate kb? The ph of a household ammonia. Web therefore, kw = ka*kb equilibrium and acid/base chemistry 1. Web this interactive quiz and printable worksheet will help you understand the concepts and definitions associated with the ka and kb of acids and. Strong acids and strong bases completely dissociate i.e. It's concentration is 0.20m and it's equilibrium concentration of h+ ions is 0.0019m. So, kb = kw / ka 2. Please show all work and answer clearly on your own paper. Name:_period:_ ka and kb calculations The ph of 0.015 m of. Calculate the ph of a. Find the ph of a solution prepared by dissolving 1.61g of nac3h3o2 in enough water to make 835ml of solution.Ka and Kb Calculations MS MCLARTY'S CLASSES
4b worksheet ka kb answers Ph Acid
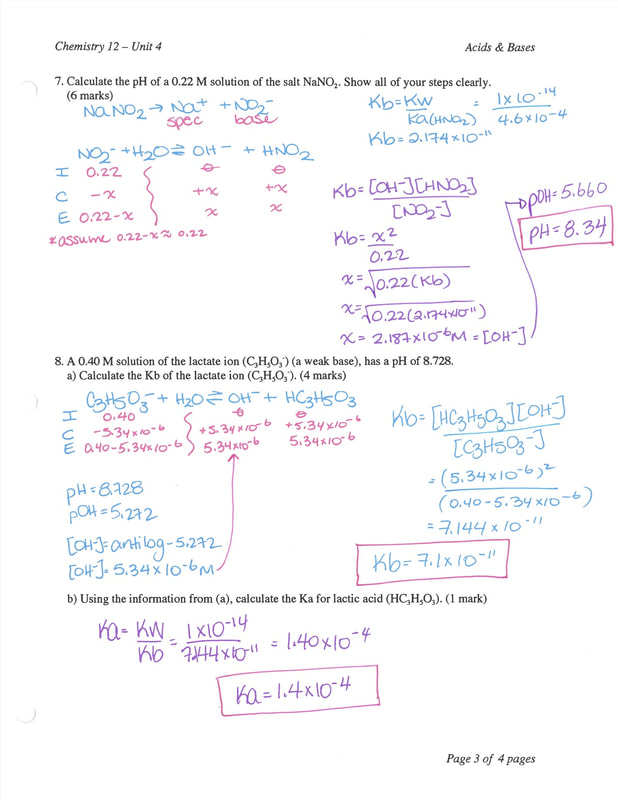
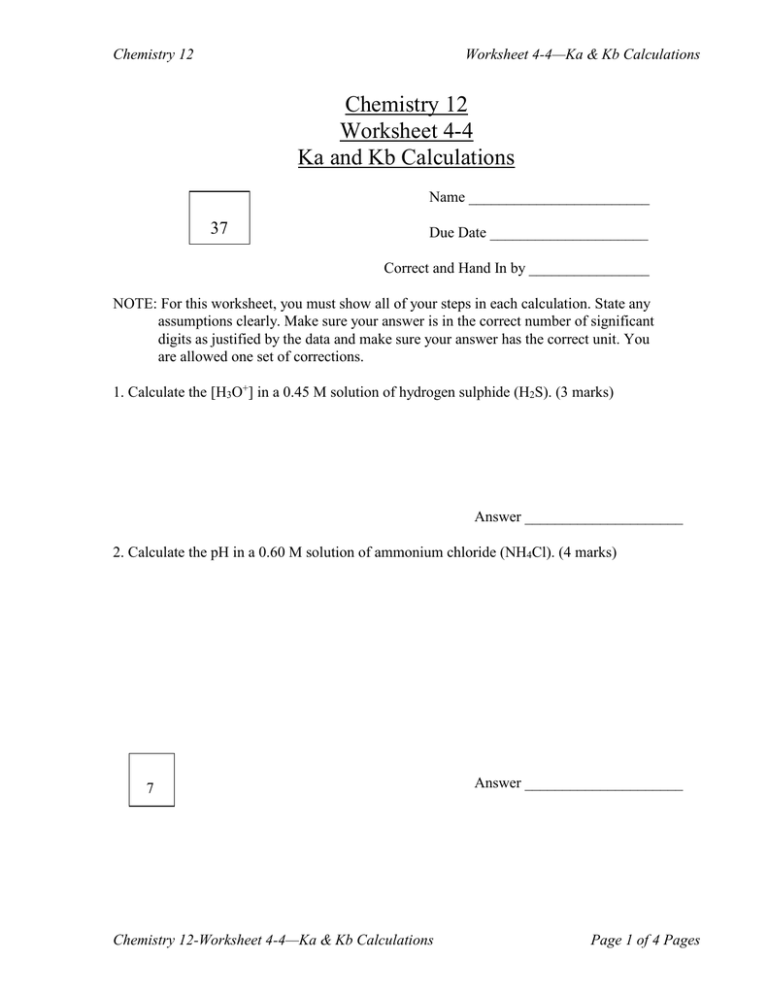
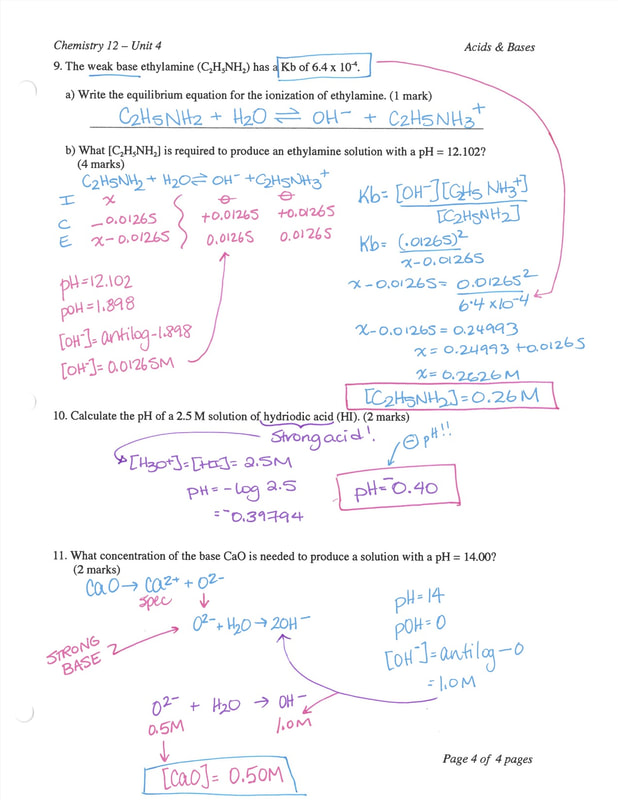
Chemistry 12 Worksheet 44 Ka and Kb Calculations 37
Ka and Kb Calculations MS MCLARTY'S CLASSES
Worksheet44 Ka & Kb Calculations key Digital …start.sd34.bc.ca
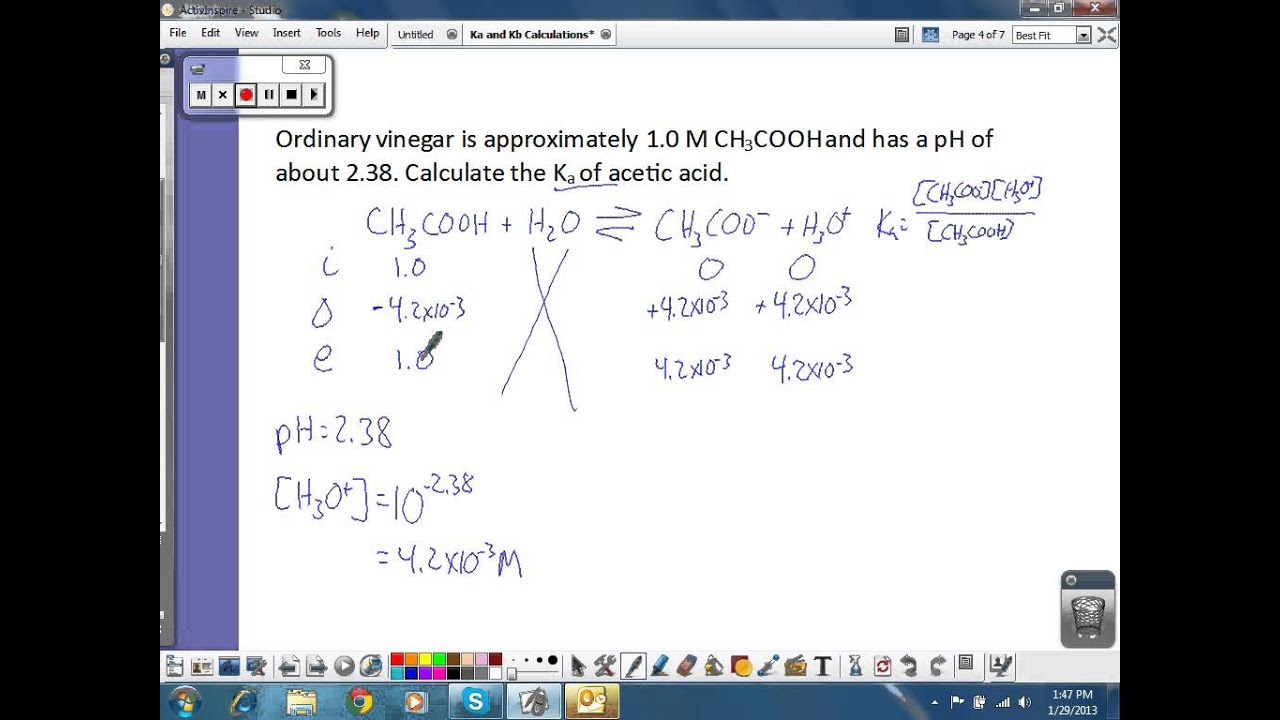
Ka and Kb Calculations YouTube
Ka and Kb Calculations MS MCLARTY'S CLASSES
Ka and Kb Calculations MS MCLARTY'S CLASSES
Ka and Kb Calculations
Quiz & Worksheet How to Calculate the Ka or Kb of a Solution
[H +] = K Ak B[Ha] B.
In Short, Diluting A Strong Acid Or Base Has A Direct Logarithmic Effect On Ph.
[H+] = K A D.
Name:_Period:_ Ka And Kb Calculations
Related Post: