Limiting And Excess Reactants Worksheet With Answers
Limiting And Excess Reactants Worksheet With Answers - Web what is the limiting reactant? How much excess is left over? 0.67 g no = 0.67 / 30 = 0.0223 mol. N2 + h2 ( nh3. O 3 + no → o 2 +no 2. Web a) which chemical is the limiting reactant? Web to determine the amounts of product (either grams or moles), you must start with the limiting reagent. What is the excess reactant? Web limiting and excess reactants online worksheet for 11. Worksheets are limiting reagent work, limiting reagents,. 2no(g) + o2 ( 2no2. If more is required, then b is the limiting. Worksheets are limiting reagent work, limiting reagents,. Use the amount that you have, not the amount you need. Web limiting and excess reactants online worksheet for 11. In one experiment 0.866 mol of. How much excess is left over? Web limiting and excess reactant. What is the limiting reactant? Worksheets are limiting reagent work, practice problems limiting. Worksheets are limiting reagent work, limiting reagents,. C) how many grams of the excess reactant will remain after the reaction is over? When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. 0.74 g o 3 = 0.74 / 48 = 0.0154 mol o 3. You can do the exercises online or download the worksheet as pdf. Web to determine the amounts of product (either grams or moles), you must start with the limiting reagent. 2 c 2h 6 + 7 o 2 4 co 2 + 6 h 2o a. You can do the exercises online or download the worksheet as pdf. Web limiting and excess reactants online worksheet for 11. What is the limiting reactant? 2 c 2h 6 + 7 o 2 4 co 2 + 6 h 2o a. N2 + h2 ( nh3. 1 mole of o 3 reacts with 1 mole of no. Web what is the limiting reagent? Web limiting and excess reactants online worksheet for 11. Determine how much one of the reactant needs of the. You can do the exercises online or download the worksheet as pdf. 1 mole of o 3 reacts with 1 mole of no. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web how do you know which of two reactants is the limiting one? Worksheets are limiting reagent work, limiting reagents,. 0.67 g no = 0.67 / 30 = 0.0223 mol. You compare the calculated amount of b to the actual amount available. Web how do you know which of two reactants is the limiting one? Web limiting and excess reactant. N2 + h2 ( nh3. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web what is the limiting reactant? How many moles of nh3 can be produced from the reaction of 28 g of n2. Use the amount that you have, not the amount you need. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web limiting and excess reagents. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Web what is the limiting reactant? Web a) which chemical is the limiting reactant? In one experiment 0.866 mol of. 1 mole of o 3 reacts with 1 mole of no. What is the excess reactant? 2 c 2h 6 + 7 o 2 4 co 2 + 6 h 2o a. 0.74 g o 3 = 0.74 / 48 = 0.0154 mol o 3. Web how do you know which of two reactants is the limiting one? All of the questions on this worksheet involve the following reaction: In one experiment 0.866 mol of. Web limiting and excess reactants online worksheet for 11. Worksheets are limiting reagent work, practice problems limiting. You compare the calculated amount of b to the actual amount available. Use the following balanced equation. Web what is the limiting reagent? Use the amount that you have, not the amount you need. 0.74 g o 3 = 0.74 / 48 = 0.0154 mol o 3. Web to determine the amounts of product (either grams or moles), you must start with the limiting reagent. 1 mole of o 3 reacts with 1 mole of no. C) how many grams of the excess reactant will remain after the reaction is over? Web worksheet on limiting reactants. Web a) which chemical is the limiting reactant? If more is required, then b is the limiting. 0.67 g no = 0.67 / 30 = 0.0223 mol. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: If 15 g of c 2h 6 react with. B) how many grams of zns will be formed? C) how many grams of the excess reactant will remain after the reaction is over? Web limiting and excess reactant. What is the limiting reactant? Can use either of the following to determine the limiting reactant. 0.67 g no = 0.67 / 30 = 0.0223 mol. You compare the calculated amount of b to the actual amount available. If 15 g of c 2h 6 react with. Worksheets are limiting reagent work, limiting reagents,. Web to determine the amounts of product (either grams or moles), you must start with the limiting reagent. Use the following balanced equation. If more is required, then b is the limiting. 2no(g) + o2 ( 2no2. Use the amount that you have, not the amount you need. You can do the exercises online or download the worksheet as pdf. Web limiting and excess reactants online worksheet for 11. How many moles of nh3 can be produced from the reaction of 28 g of n2.Limiting And Excess Reactants Worksheet Answers Askworksheet
Limiting And Excess Reactants Worksheet —
Limiting Reagent Worksheet Answers Chemical Reactions Sodium
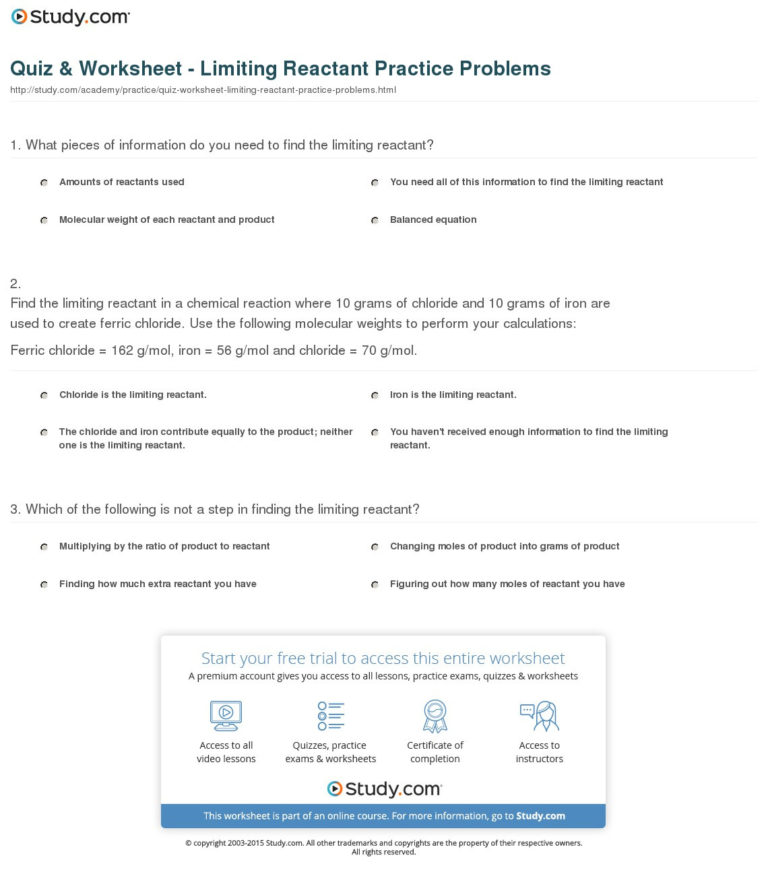
Quiz & Worksheet Limiting Reactants & Excess Reactants
W.LimitingReagentsandPercentYield.HW1.ANSWERKEY
Limiting Reactant Worksheet Answers Word Worksheet
Limiting Reagent Worksheet
Limiting Reactant Worksheet Answers Word Worksheet
Limiting Reactants Worksheet
Limiting And Excess Reactants Worksheet —
All Of The Questions On This Worksheet Involve The Following Reaction:
Web Limiting And Excess Reagents.
Nitric Oxide (No) Reacts With Oxygen Gas To Form Nitrogen Dioxide (No2), A Dark Brown Gas:
Worksheets Are Limiting Reagent Work, Practice Problems Limiting.
Related Post: