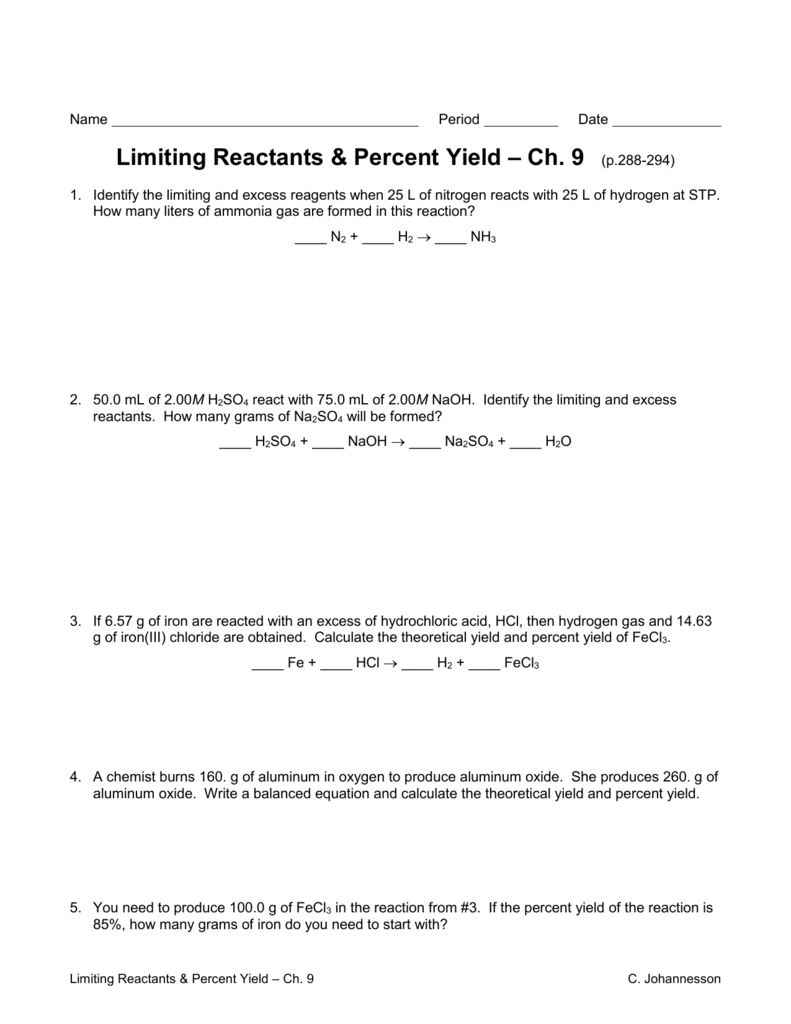
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Limiting Reactant And Percent Yield Practice Worksheet Answer Key - Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Click unit11wk4ans.doc link to view the file. Web percent yield, limiting reactants and excess reactants worksheet and answers will help and challenge students to gain a better understanding of the topic. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Calculate the percent yield for. Web practice the calculations to find the limiting reagents and yields. Limiting reagents (answer key) take the reaction: Web this worksheet and quiz will let you practice the following skills: Web the percent yield is calculated as follows: Web to identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the. Web when the amount of both reactants is given, you must determine the lr and then, based on the moles of the lr, determine the amount of product (s) that can be formed. Calculate the percent yield for. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web this worksheet and quiz will. Web percent yield, limiting reactants and excess reactants worksheet and answers will help and challenge students to gain a better understanding of the topic. Click unit11wk4ans.doc link to view the file. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. When copper (ii) chloride reacts with sodium nitrate, copper. Nh 3 + o 2 no + h 2 o. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Web explain the concepts of theoretical yield and limiting reactants/reagents. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web to identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the. Web worksheets are limiting reagent practice problems, limiting reagent work, work limiting. Web this worksheet and quiz will let you practice the following skills: Web practice the calculations to find the limiting reagents and yields. Derive the theoretical yield for a reaction under specified conditions. Web explain the concepts of theoretical yield and limiting reactants/reagents. Calculate the percent yield for. Web practice the calculations to find the limiting reagents and yields. Derive the theoretical yield for a reaction under specified conditions. Web the percent yield is calculated as follows: \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Zinc and sulphur react to form zinc sulphide according to the equation. Derive the theoretical yield for a reaction under specified conditions. Web the percent yield is calculated as follows: Click unit11wk4ans.doc link to view the file. Web this worksheet and quiz will let you practice the following skills: Nh 3 + o 2 no + h 2 o. Web this worksheet and quiz will let you practice the following skills: Web explain the concepts of theoretical yield and limiting reactants/reagents. Limiting reagents (answer key) take the reaction: In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Web practice the calculations to find the limiting reagents and yields. Web to identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or. Derive the theoretical yield for a reaction under specified conditions. Calculate the percent yield for. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Web when the amount of both reactants is given, you must determine the lr and then, based on the moles of the lr, determine the amount of. Derive the theoretical yield for a reaction under specified conditions. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web the percent yield is calculated as follows: Web percent yield, limiting reactants and excess reactants worksheet and answers will help and challenge students to gain a better understanding of the topic. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. In an experiment, 3.25 g of nh 3 are allowed to react with 3.50 g of o 2. Web when the amount of both reactants is given, you must determine the lr and then, based on the moles of the lr, determine the amount of product (s) that can be formed. Web ii) what percentage yield of iodine was produced. Web worksheets are limiting reagent practice problems, limiting reagent work, work limiting reactants name, limiting reagents, , limiting reactant and percent yield practice. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Zinc and sulphur react to form zinc sulphide according to the equation. Limiting reagents (answer key) take the reaction: Web this worksheet and quiz will let you practice the following skills: Click unit11wk4ans.doc link to view the file. Web explain the concepts of theoretical yield and limiting reactants/reagents. Nh 3 + o 2 no + h 2 o. Web to identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the. Calculate the percent yield for. Web practice the calculations to find the limiting reagents and yields. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. Nh 3 + o 2 no + h 2 o. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web practice the calculations to find the limiting reagents and yields. Click unit11wk4ans.doc link to view the file. Web worksheets are limiting reagent practice problems, limiting reagent work, work limiting reactants name, limiting reagents, , limiting reactant and percent yield practice. Calculate the percent yield for. Derive the theoretical yield for a reaction under specified conditions. Web to identify the limiting reactant, calculate the number of moles of each reactant present and compare this ratio to the mole ratio of the reactants in the. Web ii) what percentage yield of iodine was produced. Web percent yield, limiting reactants and excess reactants worksheet and answers will help and challenge students to gain a better understanding of the topic. Limiting reagents (answer key) take the reaction: Web this worksheet and quiz will let you practice the following skills: When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web the percent yield is calculated as follows:limiting reagent practice worksheet
Limiting Reactant Percent Yield Answers Key
19 Unique Limiting Reagent Worksheet 2
Limiting Reactant Worksheet Answers
41 Limiting Reactant And Percent Yield Worksheet Answer Key Worksheet
W.LimitingReagentsandPercentYield.HW1.ANSWERKEY
Limiting Factors Worksheet Answer Key Organicish
Limiting Reactant And Percent Yield Practice Worksheet Answer Key
Limiting Reactant And Percent Yield Worksheet Answer Key —
Limiting Reagents Worksheet
Web When The Amount Of Both Reactants Is Given, You Must Determine The Lr And Then, Based On The Moles Of The Lr, Determine The Amount Of Product (S) That Can Be Formed.
Zinc And Sulphur React To Form Zinc Sulphide According To The Equation.
Web Explain The Concepts Of Theoretical Yield And Limiting Reactants/Reagents.
In An Experiment, 3.25 G Of Nh 3 Are Allowed To React With 3.50 G Of O 2.
Related Post: