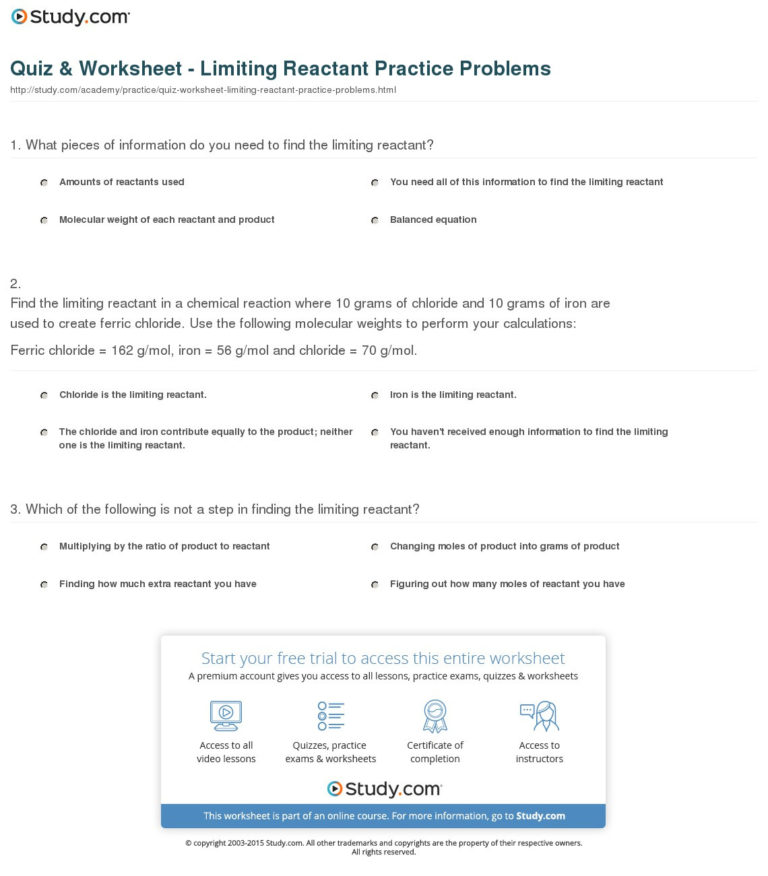
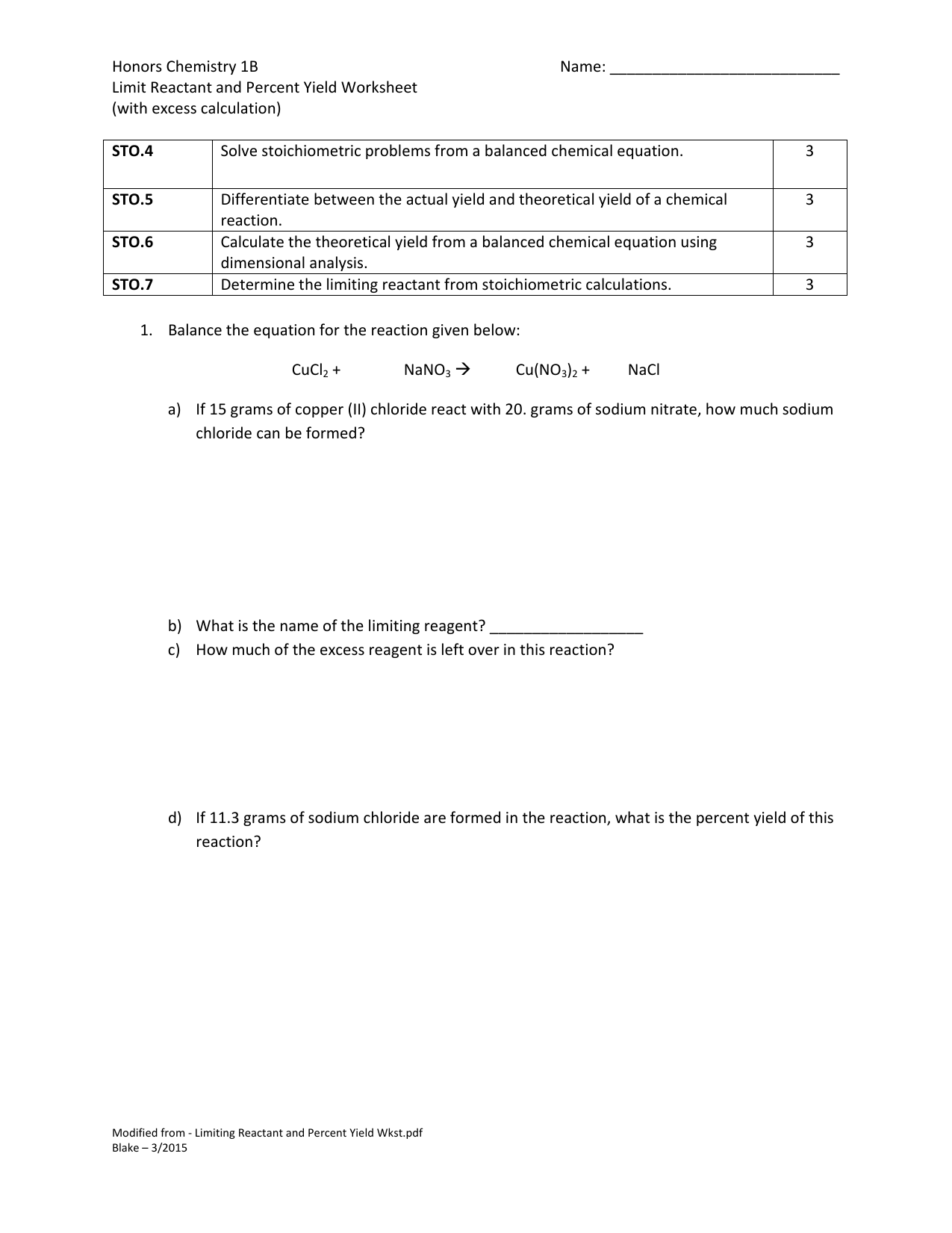
Limiting Reactant Practice Worksheet
Limiting Reactant Practice Worksheet - Web this online quiz and printable worksheet are available for you to practice what know about limiting reactions. The limiting reactant is so called as it limits the amount of product. Convert the 23 grams of sodium to moles; Multiply the ratio of product to reactant, or 1:2; D) determine the number of. Web the reactant that is not in excess is known as the limiting reactant (also known as the limiting reagent). The practice problems will help you identify and retain important. Web a) determine the limiting reagent. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. 2no(g) + o2 ( 2no2. Web the reactant that is not in excess is known as the limiting reactant (also known as the limiting reagent). This is a set of practice problems to help master the concept of limiting reactant which is critical in calculating the amount of. Limiting reactant and percent yield worksheet. Web worksheet on limiting reactants. C) determine the number of grams. Web test your knowledge of limiting reactants with this quiz and worksheet. Multiply the ratio of product to reactant, or 1:2; Web up to 24% cash back limiting reactant and percent yield practice name_____ 1) consider the following reaction: Web worksheet on limiting reactants. Web the limiting reactant (or limiting reagent) is the reactant that gets consumed first in a. This is a set of practice problems to help master the concept of limiting reactant which is critical in calculating the amount of. Web this online quiz and printable worksheet are available for you to practice what know about limiting reactions. Topics of quiz questions include calculating limiting reactants, as. D) determine the number of. Web up to 24% cash. Topics of quiz questions include calculating limiting reactants, as. B) determine the number of moles of \(h_2o\) produced. 2no(g) + o2 ( 2no2. Web test your knowledge of limiting reactants with this quiz and worksheet. Web this online quiz and printable worksheet are available for you to practice what know about limiting reactions. Web a) determine the limiting reagent. This is a set of practice problems to help master the concept of limiting reactant which is critical in calculating the amount of. D) determine the number of. The limiting reactant is so called as it limits the amount of product. N2 + h2 ( nh3. How many moles of nh3 can be produced from the reaction of 28 g of n2. Web limiting reactant and % yield worksheet. So 10/23 = 0.4 moles of hydrogen. Convert the 23 grams of sodium to moles; When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Chlorine can replace bromine in bromide compounds forming. This is a set of practice problems to help master the concept of limiting reactant which is critical in calculating the amount of. Web test your knowledge of limiting reactants with this quiz and worksheet. Convert the 23 grams of sodium to moles; Web the limiting reactant (or limiting reagent) is the. C) determine the number of grams of \(caso_4\) produced. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web limiting reagent worksheet #1 1. Web up to 24% cash back limiting reactant and percent yield practice name_____ 1) consider the following reaction: The limiting reactant is so called as it limits the amount. B) determine the number of moles of \(h_2o\) produced. D) determine the number of. Web worksheet 14 3 answers to worksheet #14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Convert the 23 grams of sodium. Students need to determine the limiting reactant and the percent yield of each reaction. So 0.4 (1/2) = 0.2 moles of hydrogen. So 10/23 = 0.4 moles of hydrogen. Topics of quiz questions include calculating limiting reactants, as. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web this worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. C) determine the number of grams of \(caso_4\) produced. D) determine the number of. So 10/23 = 0.4 moles of hydrogen. How many moles of nh3 can be produced from the reaction of 28 g of n2. Web the limiting reactant (or limiting reagent) is the reactant that gets consumed first in a chemical reaction and therefore limits how much product can be formed. So 0.4 (1/2) = 0.2 moles of hydrogen. This reagent is the one that determines. N2 + h2 ( nh3. 2no(g) + o2 ( 2no2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web worksheet 14 3 answers to worksheet #14 limiting reagents a limiting reagent is the reactant that is completely used up in a reaction. Web worksheet on limiting reactants. Web this online quiz and printable worksheet are available for you to practice what know about limiting reactions. In one experiment 0.866 mol of. Nh 4 no 3 + na 3 po 4 (nh 4) 3 po 4 + nano 3 which reactant is. Students need to determine the limiting reactant and the percent yield of each reaction. The limiting reactant is so called as it limits the amount of product. Topics of quiz questions include calculating limiting reactants, as. Web limiting reactant and % yield worksheet. Students need to determine the limiting reactant and the percent yield of each reaction. Nh 4 no 3 + na 3 po 4 (nh 4) 3 po 4 + nano 3 which reactant is. Web the reactant that is not in excess is known as the limiting reactant (also known as the limiting reagent). This is a set of practice problems to help master the concept of limiting reactant which is critical in calculating the amount of. N2 + h2 ( nh3. C) determine the number of grams of \(caso_4\) produced. Limiting reactant and percent yield worksheet. How many moles of nh3 can be produced from the reaction of 28 g of n2. Topics of quiz questions include calculating limiting reactants, as. Nitric oxide (no) reacts with oxygen gas to form nitrogen dioxide (no2), a dark brown gas: Web limiting reagent worksheet #1 1. Web limiting reactant and % yield worksheet. So 10/23 = 0.4 moles of hydrogen. This reagent is the one that determines. 2no(g) + o2 ( 2no2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed.Quiz Worksheet Limiting Reactant Practice Problems —
Limiting Reactant Worksheet Answers
limiting reagent practice worksheet
Quiz & Worksheet Limiting Reactant
Limiting Reactants Worksheet
Limiting Reactant Problems Worksheet —
Limiting Reactant Worksheet Answers Word Worksheet
Limiting Reactant Worksheet Answers Beautiful Limiting Reagent
Stoichiometry Limiting Reagent Worksheet
Limiting Reactant Worksheet 2 —
Web This Worksheet Provides Ten Examples For Students To Work Through The Processes Of Determining The Limiting Reactant, Theoretical Yield, And/Or The Percent Yield Of A.
So 0.4 (1/2) = 0.2 Moles Of Hydrogen.
Chlorine Can Replace Bromine In Bromide Compounds Forming.
Web Worksheet 14 3 Answers To Worksheet #14 Limiting Reagents A Limiting Reagent Is The Reactant That Is Completely Used Up In A Reaction.
Related Post: