Limiting Reactant Worksheet 1
Limiting Reactant Worksheet 1 - How is the limiting reactant identified? D) determine the number of. Web a) determine the limiting reagent. D) 13.86 g c3h8 2a) al2(so3)3. B) determine the number of moles of \(h_2o\) produced. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. Balance the equation first) c3h8 + o2 g co2 + h2o. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. N2 + h2 ( nh3. Web up to 24% cash back chemistry worksheet: 2 nacl (aq)+ h2so4 (aq) na2so4 (aq)+ 2 hcl (aq) if. N2 + h2 ( nh3. D) determine the number of. How many moles of nh3 can be produced from the reaction of 28 g of n2. Web limiting reactants worksheet 1. Web this worksheet set guides students through the following topics:what is a limiting reactant in a chemical reaction? Web up to 24% cash back chemistry worksheet: Web the limiting reactant (or limiting reagent) is the reactant that gets consumed first in a chemical reaction and therefore limits how much product can be formed. 2 al + 6 hbr → 2. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. D) determine the number of. This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web a limiting reactant is. How many moles of nh3 can be produced from the reaction of 28 g of n2. Web a) determine the limiting reagent. C) determine the number of grams of \(caso_4\) produced. Ko2 maximum or theoretical yield = 0.11 mol o2. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. Web a limiting reactant is a component in a chemical reaction that is consumed first, restricting the amount of product that can be formed. How many moles of nh3 can be produced from the reaction of 28 g of n2. Start with a balanced chemical equation. 2 nacl (aq)+ h2so4 (aq) na2so4 (aq)+ 2 hcl (aq) if. When copper (ii). How is the limiting reactant identified? How many moles of nh3 can be produced from the reaction of 28 g of n2. All of the questions on this worksheet involve the following reaction: Web a) determine the limiting reagent. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8. Web 0.10 mol h2o x 3 mol o2 = 0.15 mol o2. How many moles of nh3 can be produced from the reaction of 28 g of n2. C) determine the number of grams of \(caso_4\) produced. Web up to 24% cash back chemistry worksheet: (hint balance the equation first) c 3h 8 + o 2 co 2 + h. Balance the equation first) c3h8 + o2 g co2 + h2o. 2 al + 6 hbr → 2 albr3+ 3 h2. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web worksheet on limiting reactants. Web up to 24% cash back chemistry worksheet: Convert any amount given (for example in grams). Web 0.10 mol h2o x 3 mol o2 = 0.15 mol o2. B) determine the number of moles of \(h_2o\) produced. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. Balance the equation first) c3h8 + o2 g co2 + h2o. How many moles of nh3 can be produced from the reaction of 28 g of n2. Web up to 24% cash back chemistry worksheet: If you start with 14.8 g of c3h8 and 3.44 g of. Web 0.10 mol h2o x 3 mol o2 = 0.15 mol o2. Balance the equation first) c3h8 + o2 g co2 + h2o. C) determine the number of grams of \(caso_4\) produced. Convert any amount given (for example in grams). This worksheet provides ten examples for students to work through the processes of determining the limiting reactant, theoretical yield, and/or the percent yield of a. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8 and 3.44 g o 2 a). 2 nacl (aq)+ h2so4 (aq) na2so4 (aq)+ 2 hcl (aq) if. Web this worksheet set guides students through the following topics:what is a limiting reactant in a chemical reaction? Balance the equation first) c3h8 + o2 g co2 + h2o. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. D) determine the number of. Limiting reactant worksheet #1 1. If you start with 14.8 g of c3h8 and 3.44 g of. 2 al + 6 hbr → 2 albr3+ 3 h2. Web a) determine the limiting reagent. Some of the worksheets for this concept are limiting reagent work, practice problems limiting. Ko2 maximum or theoretical yield = 0.11 mol o2. How many moles of nh3 can be produced from the reaction of 28 g of n2. N2 + h2 ( nh3. D) 13.86 g c3h8 2a) al2(so3)3. B) determine the number of moles of \(h_2o\) produced. Start with a balanced chemical equation. Web a limiting reactant is a component in a chemical reaction that is consumed first, restricting the amount of product that can be formed. A) if you start with 14 g of c 3 h. C) determine the number of grams of \(caso_4\) produced. 2 al + 6 hbr → 2 albr3+ 3 h2. When 3.22 moles of al reacts with. Balance the equation first) c3h8 + o2 g co2 + h2o. Web this worksheet set guides students through the following topics:what is a limiting reactant in a chemical reaction? All of the questions on this worksheet involve the following reaction: Start with a balanced chemical equation. Web worksheet on limiting reactants. (hint balance the equation first) c 3h 8 + o 2 co 2 + h 2o if you start with 14.8 g of c 3h 8 and 3.44 g o 2 a). D) 13.86 g c3h8 2a) al2(so3)3. Ko2 maximum or theoretical yield = 0.11 mol o2. Web 0.10 mol h2o x 3 mol o2 = 0.15 mol o2. 2 nacl (aq)+ h2so4 (aq) na2so4 (aq)+ 2 hcl (aq) if. Some of the worksheets for this concept are limiting reagent work, practice problems limiting.chemistry worksheet limiting reactant worksheet 1
Limiting Reagent Worksheet 1
Limiting Reactant and percent yield worksheets
Worksheet Stoichiometry Limiting Reactants Ennature
limiting reagent practice worksheet
Limiting Reagent Worksheet 1 Answers trendstoday
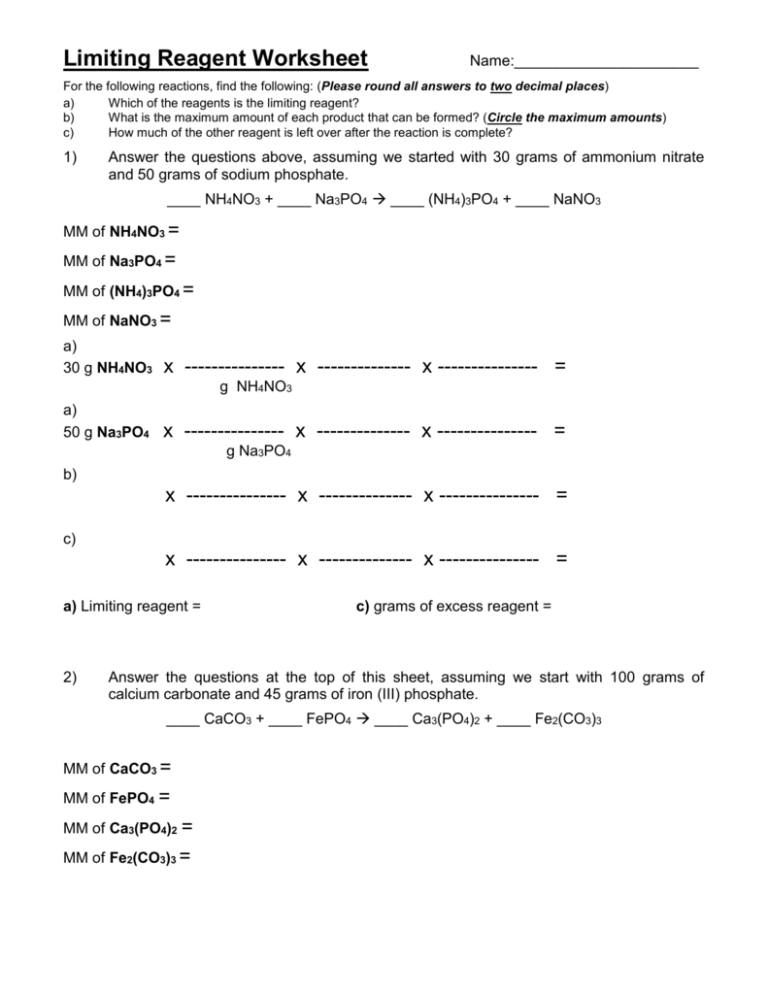
Limiting Reagent Worksheet
Limiting Reactant Worksheet
Limiting Reagent Worksheet Answer Key With Work —
Limiting Reactant Worksheets Answer Key
This Worksheet Provides Ten Examples For Students To Work Through The Processes Of Determining The Limiting Reactant, Theoretical Yield, And/Or The Percent Yield Of A.
Web Limiting Reactants Worksheet 1.
Web The Limiting Reactant (Or Limiting Reagent) Is The Reactant That Gets Consumed First In A Chemical Reaction And Therefore Limits How Much Product Can Be Formed.
Limiting Reactant Worksheet #1 1.
Related Post: