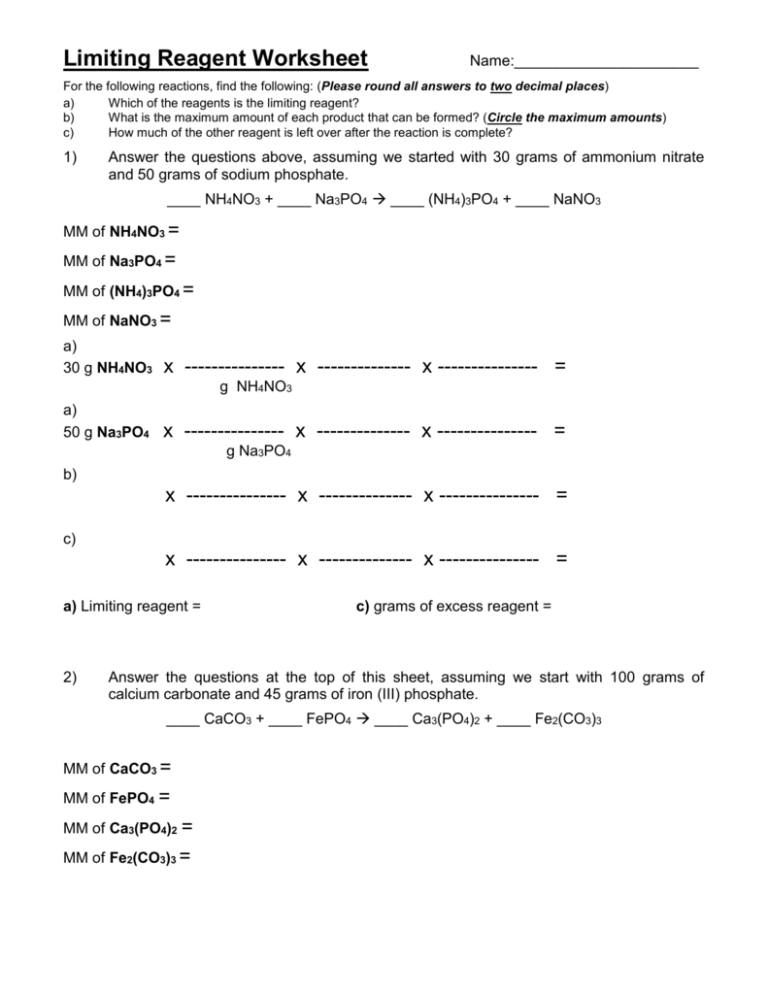
Limiting Reagent And Percent Yield Worksheet
Limiting Reagent And Percent Yield Worksheet - Practice the calculations to find the limiting reagents and yields. Each worksheet has two different chemical equations. 3) how much of the excess reagent remains when this reaction has gone to completion? All of the questions on this worksheet involve the following reaction: Web phosphate, what is the limiting reagent? Each chemical equation comes with 2 limiting reagent calculations and one percent yield. Web two worksheets are included. Web what was the percent yield? This is the reagent that is completely. Web in this worksheet, we will practice identifying the limiting reagent and calculating the percentage yield of desired products based on the actual and theoretical yield. Web understanding limiting and excess reagents. This is the reagent that is completely. Web whenever quantities of two or more reactants are given in a stoichiometric problem, you must identify the _________. Limiting reactant and percent yield worksheet. Web limiting reactant and percentage yield. Web the limiting reagent is n 2. Each chemical equation comes with 2 limiting reagent calculations and one percent yield. This is the reagent that is completely. Predict quantities of products produced or reactants consumed based on complete consumption of limiting reagent (on both mole. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are. Web in this worksheet, we will practice identifying the limiting reagent and calculating the percentage yield of desired products based on the actual and theoretical yield. Web two worksheets are included. Web after learning how to solve stoichiometric problems, this is an introduction to the application of that process for both determining the limiting reagent and percent yield. Web phosphate,. Web based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. 12 g is the theoretical yield 8.25 g is the actual yield. Calculate the percent yield by dividing the actual yield. Web view limiting reagents and percentage yield worksheet.docx from chemistry 233 at university of chicago. Limiting reactant and percent yield. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Web phosphate, what is the limiting reagent? 12 g is the theoretical yield 8.25 g is the actual yield. Web the limiting reagent is n 2. Web view limiting reagents and percentage yield worksheet.docx from chemistry 233 at university of chicago. Web the percent yield is calculated as follows: Web understanding limiting and excess reagents. Web what was the percent yield? _____ limiting reagent and percent yield 1. How much sodium chloride can be formed? Web whenever quantities of two or more reactants are given in a stoichiometric problem, you must identify the _________. Web limiting reactant and percentage yield. All of the questions on this worksheet involve the following reaction: Web in this worksheet, we will practice identifying the limiting reagent and calculating the percentage yield of desired products based on the actual and. Each chemical equation comes with 2 limiting reagent calculations and one percent yield. Web the percent yield is calculated as follows: How much sodium chloride can be formed? Web limiting reactant and percentage yield. Name_ date_ period_ limiting reagents and percentage yield. Web the percent yield is calculated as follows: 12 g is the theoretical yield 8.25 g is the actual yield. Web after learning how to solve stoichiometric problems, this is an introduction to the application of that process for both determining the limiting reagent and percent yield. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\%. Limiting reactant and percent yield worksheet. Web what was the percent yield? Web the percent yield is calculated as follows: When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. Calculate the percent yield by dividing the actual yield. 3) how much of the excess reagent remains when this reaction has gone to completion? The percent yield is based upon the theoretical yield. Name_ date_ period_ limiting reagents and percentage yield. Web after learning how to solve stoichiometric problems, this is an introduction to the application of that process for both determining the limiting reagent and percent yield. Web view limiting reagents and percentage yield worksheet.docx from chemistry 233 at university of chicago. Practice the calculations to find the limiting reagents and yields. Web based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. Predict quantities of products produced or reactants consumed based on complete consumption of limiting reagent (on both mole. Web limiting reactant and percentage yield. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. _____ limiting reagent and percent yield 1. How much sodium chloride can be formed? Web phosphate, what is the limiting reagent? Web two worksheets are included. 12 g is the theoretical yield 8.25 g is the actual yield. Each chemical equation comes with 2 limiting reagent calculations and one percent yield. This is the reagent that is completely. Web the limiting reagent is n 2. Calculate the percent yield by dividing the actual yield. Web limiting reagent and percent yield worksheet name period 1. Web view limiting reagents and percentage yield worksheet.docx from chemistry 233 at university of chicago. Web understanding limiting and excess reagents. Web limiting reagent and percent yield worksheet name period 1. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate and sodium chloride are formed. \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web in this worksheet, we will practice identifying the limiting reagent and calculating the percentage yield of desired products based on the actual and theoretical yield. Practice the calculations to find the limiting reagents and yields. How much sodium chloride can be formed? Chlorine can replace bromine in bromide compounds forming a chloride compound and elemental. Limiting reactant and percent yield worksheet. Web limiting reactant and percentage yield. Web phosphate, what is the limiting reagent? All of the questions on this worksheet involve the following reaction: Each worksheet has two different chemical equations. _____ limiting reagent and percent yield 1. 3) how much of the excess reagent remains when this reaction has gone to completion?Limiting reactant and percentage yield worksheet
Limiting Reagent And Percent Yield Worksheet Abjectleader
Limiting Reactant Worksheet Answers Chemistry Percent Yield Worksheet
Limiting Reagent Worksheet 2 worksheet
12 Fab Limiting Reactant And Percent Yield Worksheet Coloring Page Ee
Limiting Reactant and percent yield worksheets
Limiting Reagent Worksheet 1 YouTube
Stoichiometry, Percent Yield and Limiting Reagents practice problems
Limiting Reagent Worksheet 1 Answers trendstoday
92b Limiting Reactant and Percent Yield Worksheet
Web Whenever Quantities Of Two Or More Reactants Are Given In A Stoichiometric Problem, You Must Identify The _________.
Web Two Worksheets Are Included.
Name_ Date_ Period_ Limiting Reagents And Percentage Yield.
The Percent Yield Is Based Upon The Theoretical Yield.
Related Post: