Molarity And Dilution Worksheet
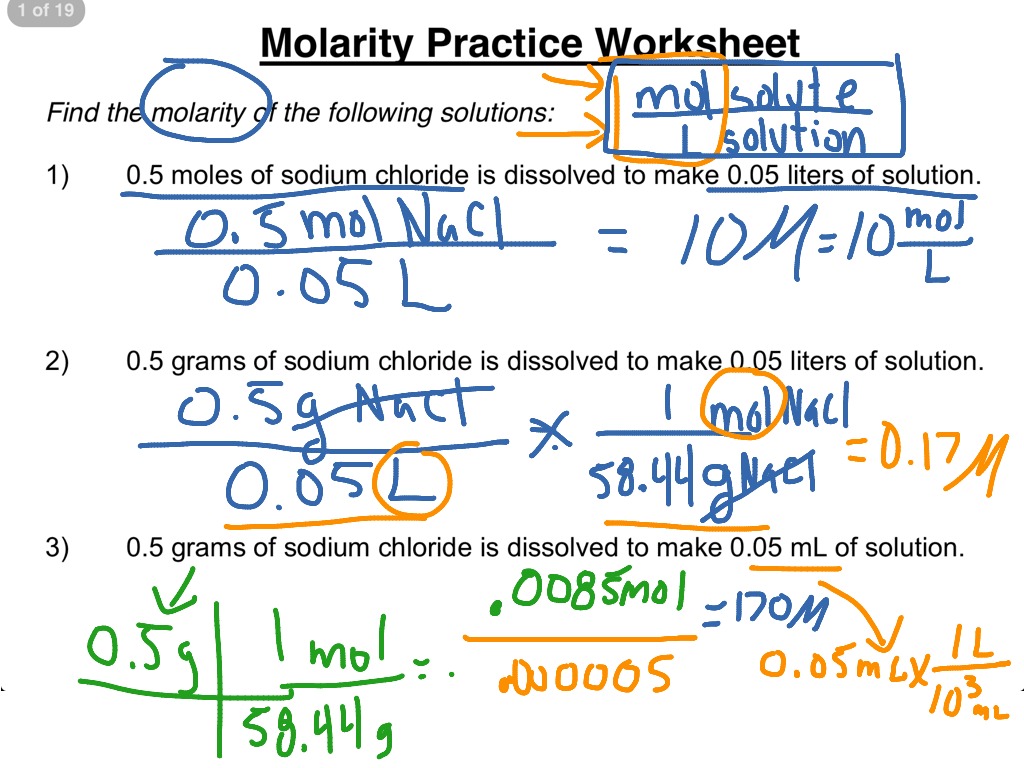
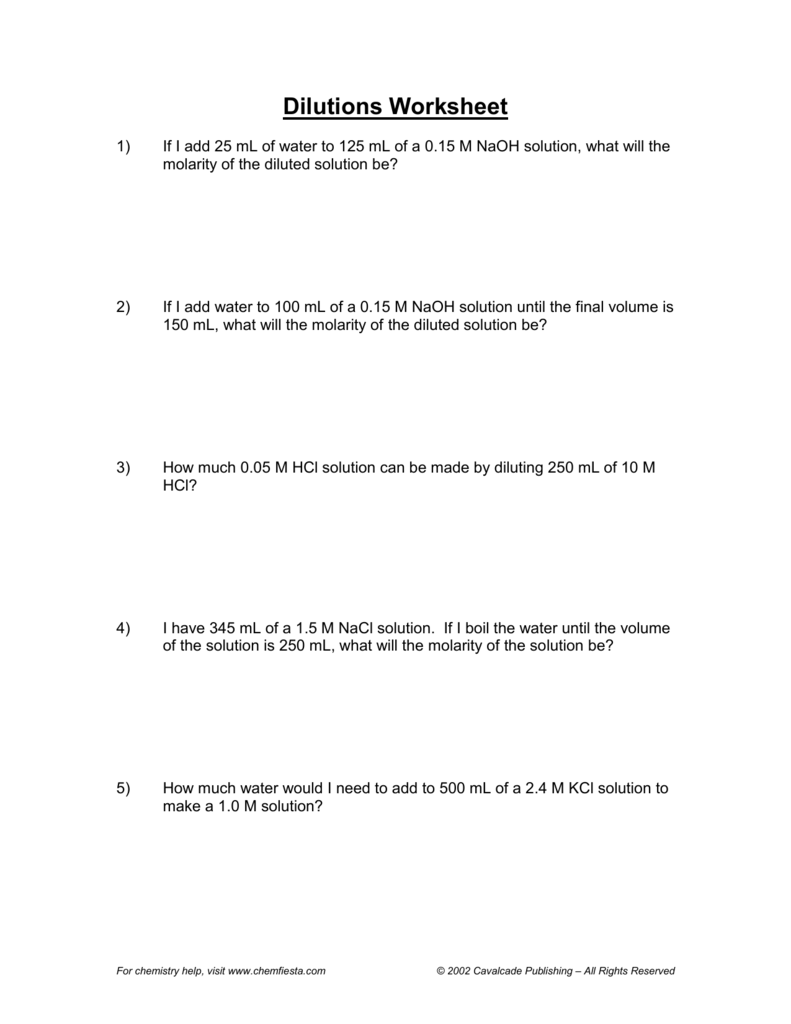
Molarity And Dilution Worksheet - 2) if i add water to 100 ml of a 0.15 m naoh. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. What is concentration and molarity? Web molarity and dilutions worksheet 1. Web molarity and dilutions worksheet. This worksheet set guides students through the following topics:what is. Calculate molarity by dissolving 25.0g naoh in 325 ml of solution. Molarity and dilution practice for chemistry. In this worksheet we will use molarity formula and dilution formula. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Web this worksheet set guides students through the following topics: What is the molarity. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume. Web this worksheet set guides students through the following topics: Web up to 24% cash back concentrations and dilutions worksheet a way of expressing concentration is called molarity. Calculate grams of solute needed to. If needed, you can. Web molarity and dilutions worksheet. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. What is the molarity of a 0 liter solution. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution.. Web chemistry ii worksheet name molarity, & dilution instructions: What is concentration and molarity? Web molarity and dilutions worksheet 1. These are the same directions that were on dilutions practice sheet. Work each of the following problems in the space provided. Web molarity and dilutions worksheet 1. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. What is the molarity of a 0 liter solution. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume.. What is the molarity of a 0 liter solution. Calculate grams of solute needed to. Web up to 24% cash back concentrations and dilutions worksheet a way of expressing concentration is called molarity. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Molarity and dilution practice. Work each of the following problems in the space provided. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate grams of solute needed to. Web up to 24% cash back concentrations and dilutions worksheet a way of expressing concentration is called molarity. Calculate the final concentration of a solution that is made by dissolving. Molarity and dilution practice for chemistry. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume. Calculate grams of solute needed to. Web this worksheet set guides students through the following topics: Web chemistry ii worksheet name molarity, & dilution instructions: 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Molarity and dilution practice for chemistry. What is the molarity of a 0 liter solution. These are the same directions that were on dilutions practice sheet. Web molarity and dilutions worksheet. These are the same directions that were on dilutions practice sheet. Work each of the following problems in the space provided. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Web molarity and dilutions worksheet 1. Web molarity and dilutions worksheet 1. Web molarity and dilutions worksheet 1. Molarity and dilution practice for chemistry. If needed, you can find the molarity of a solution by the. Web this worksheet set guides students through the following topics: This worksheet set guides students through the following topics:what is. Work each of the following problems in the space provided. In this worksheet we will use molarity formula and dilution formula. Show all your work and circle your final. Web up to 24% cash back concentrations and dilutions worksheet a way of expressing concentration is called molarity. As is clear from its name, molarity involves moles. Web molarity and dilutions worksheet 1. Calculating molarity using the molarity equation; Calculate grams of solute needed to. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. 1) if i add 25 ml of water to 125 ml of a 0.15 m naoh solution, what will the molarity of the diluted solution be? Web molarity and dilutions worksheet. Web teach your students about calculating molarity and dilutions using this great, detailed set of worksheets! Molarity and dilution practice for chemistry. This worksheet set guides students through the following topics:what is. In this worksheet we will use molarity formula and dilution formula. Web molarity and dilutions worksheet 1. Work each of the following problems in the space provided. Calculate the final concentration of a solution that is made by dissolving 14.8 g of solid sodium hydroxide in 600.0 ml of solution. Calculating molarity using the molarity equation; Web molarity and dilutions worksheet 1. These are the same directions that were on dilutions practice sheet. As is clear from its name, molarity involves moles. Web calculate molarity if 25.0 ml of 1.75 m hcl diluted to 65.0 ml. Web chemistry ii worksheet name molarity, & dilution instructions: Show all your work and circle your final. Web m1 and v1 are the initial solution’s molarity and volume, while m2 and v2 are the final solution’s molarity and volume. Web molarity and dilutions worksheet. Web this worksheet set guides students through the following topics:Molarity and Dilution Worksheet YouTube
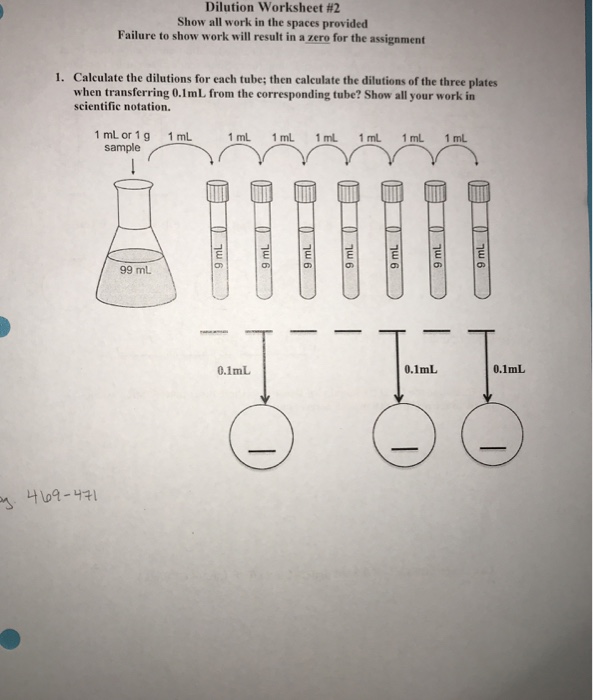
dilution calculations worksheet
️Dilutions Worksheet Answers Free Download Goodimg.co
Molarity And Dilution Worksheet Free Printable Worksheets
7 Best Images of Molarity Worksheet With Answers Molality and
Molarity And Dilution Worksheet kidsworksheetfun
7 Molarity Worksheet With Answers /
__LINK__ Molarity By Dilution Worksheet Answers Chemistry If8766
Molarity And Dilution Worksheet kidsworksheetfun
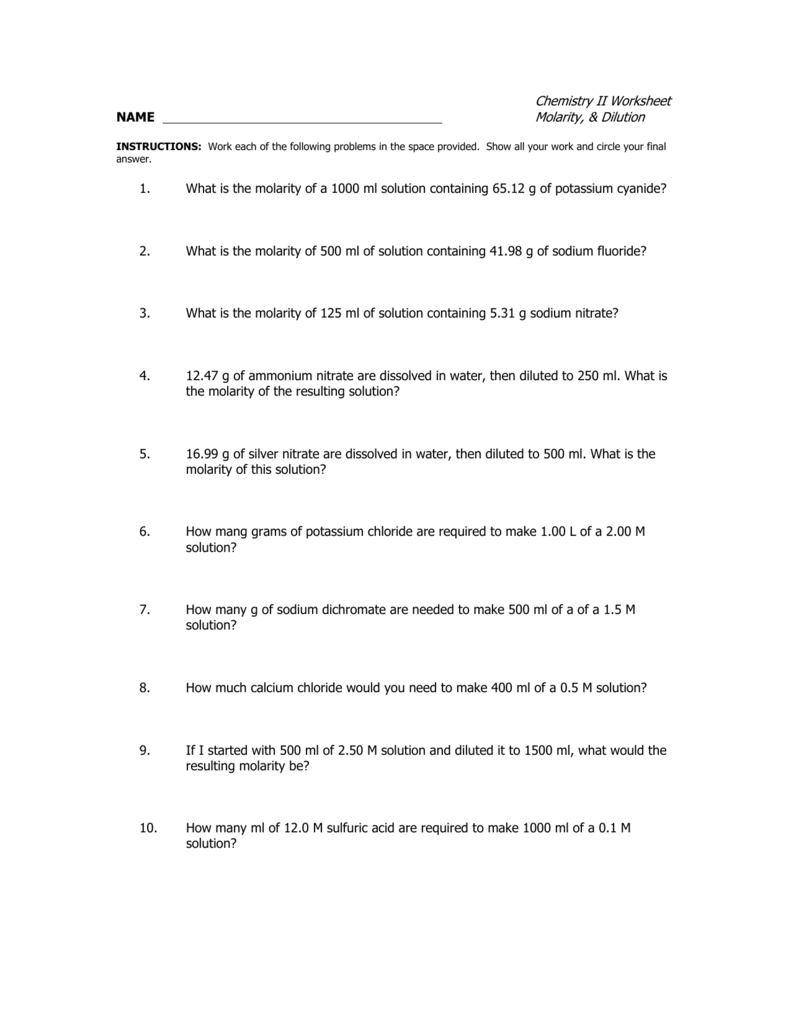
Chemistry II Worksheet NAME Molarity, & Dilution 1. What is the
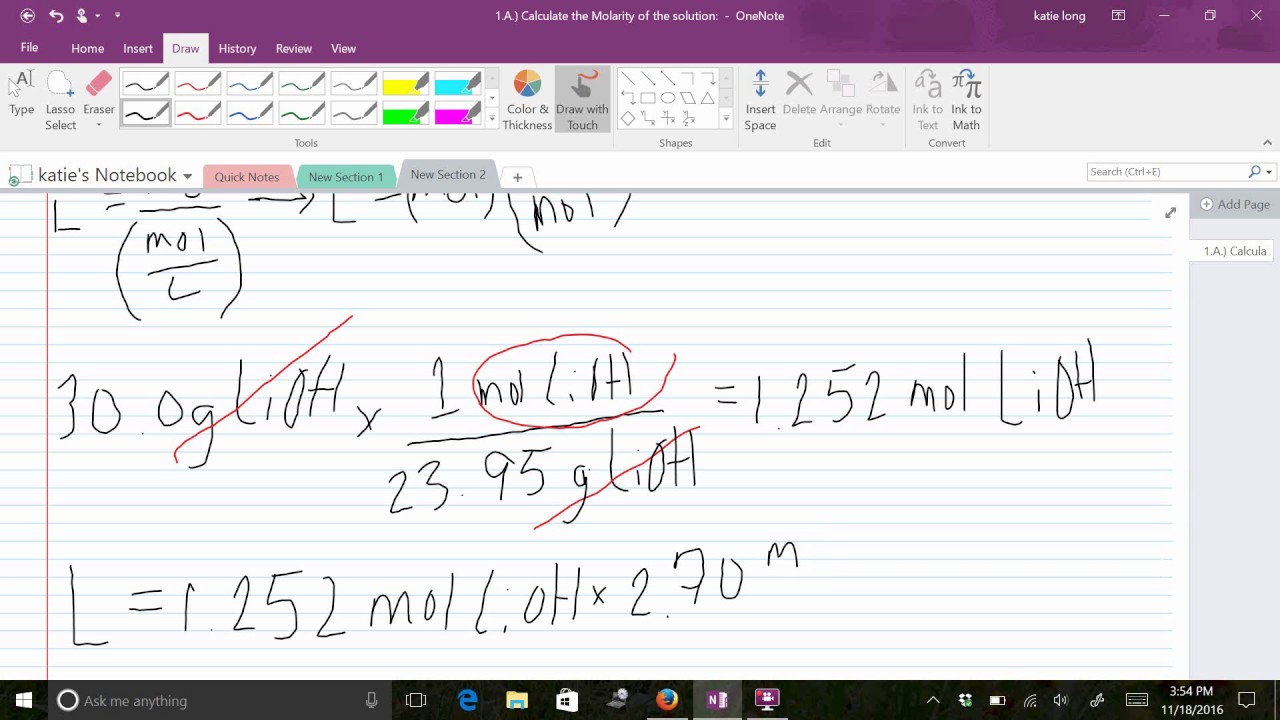
Calculate The Final Concentration Of A Solution That Is Made By Dissolving 14.8 G Of Solid Sodium Hydroxide In 600.0 Ml Of Solution.
What Is The Molarity Of A 0 Liter Solution.
Calculate Grams Of Solute Needed To.
Calculate The Final Concentration Of A Solution That Is Made By Dissolving 14.8 G Of Solid Sodium Hydroxide In 600.0 Ml Of Solution.
Related Post: