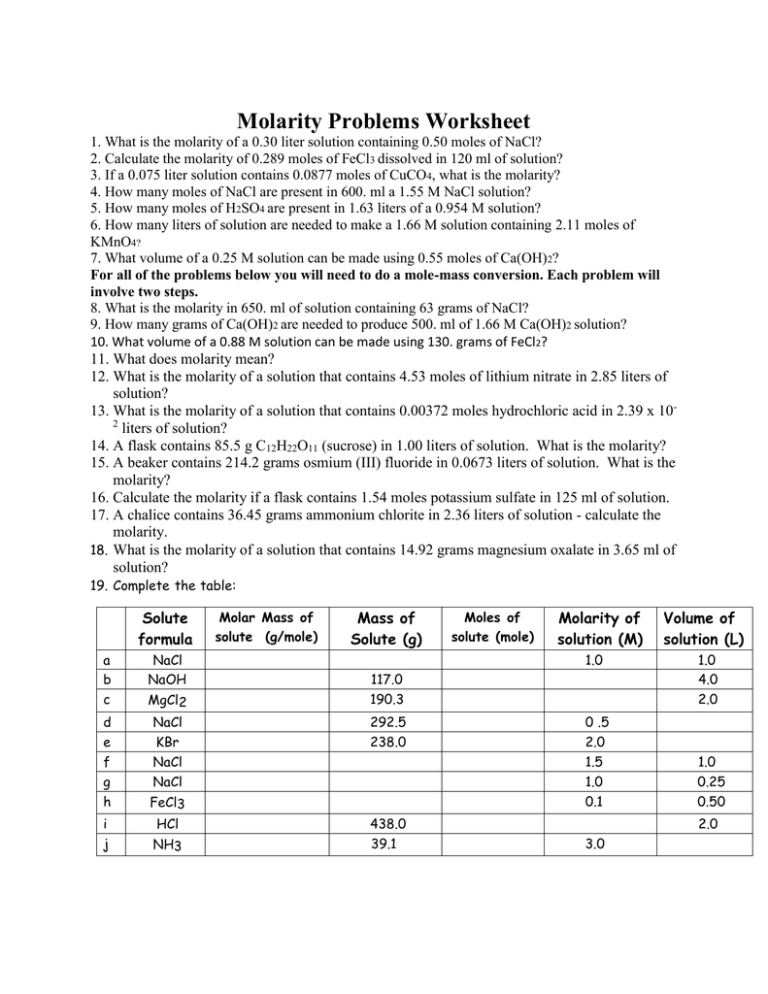
Molarity Problems Worksheet
Molarity Problems Worksheet - What is the molarity of a 0 liter solution. Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. The molarity of a solutio n is defined as the moles of solute per liter of solution. Web this worksheet focuses on the equation molarity = moles solute/liters solution.10 problems are included that ask students to solve for molarity, moles, volume, or mass. What is the molarity of sodium chloride in sea water? Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? Web what is the molarity of the solution? Do not confuse m, l, and ml! When the solvent is water, we have an aqueous solution. What is the molarity of a solution containing 325 g of nacl. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. When the solvent is water, we have an aqueous solution. How many liters of water are needed to make a. Web molarity problems worksheet use m or mol/l as unit for molarity. Remember that 1 liter = 1000 ml. Web what is the molarity of the solution? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Web molarity problems worksheet use m or mol/l as unit for molarity. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. Molarity is abbreviated as m. Web what is the molarity of the solution? 30 ml of 0.5m h 2 so 4 diluted to 500 ml. Web molarity practice problems #1 1. How many liters of water are needed to make a. Do not confuse m, l, and ml! To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? When the solvent is water, we have an aqueous solution. Web molarity = _____ problems: 30 g of co (no 3) 2.6h 2 o in 4.3l. Web molarity practice problems #1 1. Web molarity = _____ problems: Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. Mv = grams / molar mass (x) (1.00 l) = 28.0 g /. How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? Mv = grams / molar mass (x) (1.00 l) = 28.0 g /. Web molarity practice problems #1 1. Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? Remember that 1 liter = 1000 ml. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? What is the molarity of a 0 liter solution. Web what is the molarity of the solution? Molarity is abbreviated as m. To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. 30 ml of 0.5m h 2 so 4 diluted to 500 ml. The molarity of a solutio n is defined as the moles of solute per liter of solution. Web molarity problems worksheet use m or mol/l as unit for molarity. Mv = grams / molar mass (x) (1.00 l) = 28.0 g /. Calculate the molarity of a solution. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; How many grams of potassium carbonate are needed to make 280 ml of a 2.5 m solution? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. What is the molarity of a 0 liter solution. What. Web molarity practice problems #1 1. The molarity of a solutio n is defined as the moles of solute per liter of solution. Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? G kno 3 = 0.175 mol kno 3 × 101.1 g kno 3 1 mol kno 3 = 17.7 g kno 3. Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Show all work and circle your final answer. Web this worksheet focuses on the equation molarity = moles solute/liters solution.10 problems are included that ask students to solve for molarity, moles, volume, or mass. Sea water contains roughly 28.0 g of nacl per liter. G kno 3 = 0.175 mol kno 3 × 101.1 g kno 3 1 mol kno 3 = 17.7 g kno 3 m = 3.5 mol 0.125 l = 28 m 6. What is the molarity of a 0.30 liter solution containing 0.50 moles of nacl? Web molarity problems worksheet use m or mol/l as unit for molarity. How many liters of water are needed to make a. The molarity of a solutio n is defined as the moles of solute per liter of solution. Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? Mv = grams / molar mass (x) (1.00 l) = 28.0 g /. Slotsky chemistry ii molarity problems worksheet 7n m = _ where n is number of moles and v is volume in liters use m or mol/l as unit for molarity. When the solvent is water, we have an aqueous solution. Web what is the molarity of the solution? Do not confuse m, l, and ml! Remember that 1 liter = 1000 ml. Web molarity = _____ problems: Calculate the molarity of a solution which contains 0.40 mol of a substance dissolved in 1.6 l of a solution. Molarity is abbreviated as m. What is the molarity of a solution containing 325 g of nacl. When the solvent is water, we have an aqueous solution. Web what is the molarity of the solution? 30 ml of 0.5m h 2 so 4 diluted to 500 ml. Web this worksheet focuses on the equation molarity = moles solute/liters solution.10 problems are included that ask students to solve for molarity, moles, volume, or mass. Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. Mv = grams / molar mass (x) (1.00 l) = 28.0 g /. Calculate the molarity of each of the following solutions: Sea water contains roughly 28.0 g of nacl per liter. What is the molarity of sodium chloride in sea water? To make a 4.00 m solution, how many moles of solute will be needed if 12.0 liters of solution are. The molarity of a solutio n is defined as the moles of solute per liter of solution. Web molarity practice problems #1 1. 30 g of co (no 3) 2.6h 2 o in 4.3l of solution; Calculate the molarity of 0.289 moles of fecl 3 dissolved in 120 ml of solution? Show all work and circle your final answer. What is the molarity of a 0 liter solution.Molarity Worksheet Answer Key
Molarity Problems Worksheet
molarity practice worksheet Molarity Practice Worksheet . Molarity
Molarity Problems Worksheet
Molarity Practice Worksheet
Worksheets Stationery Article Molarity Problems Worksheet Answers
Molarity Practice Worksheet Answer
Molarity Calculations Worksheet Answers worksheet
Molarity Worksheet 1 worksheet
Molarity Worksheet Answer Key
What Is The Molarity Of A Solution Containing 325 G Of Nacl.
Web This Worksheet Features 5 Molarity Problems (M=Mol/L) With Conversions From Grams To Moles And Milliliters To Liters And 7 Dilutions Problems Using M1V1=M2V2.
Web What Determines The Concentration Of A Solution?
What Is The Molarity Of A 0.30 Liter Solution Containing 0.50 Moles Of Nacl?
Related Post: