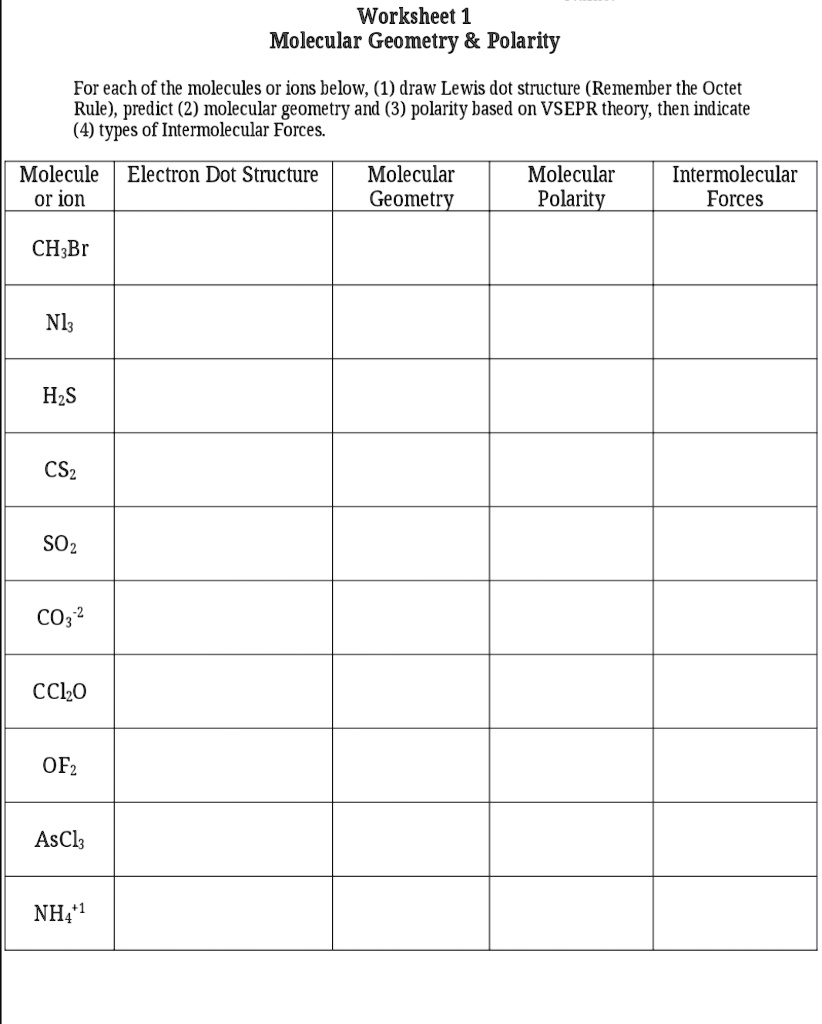
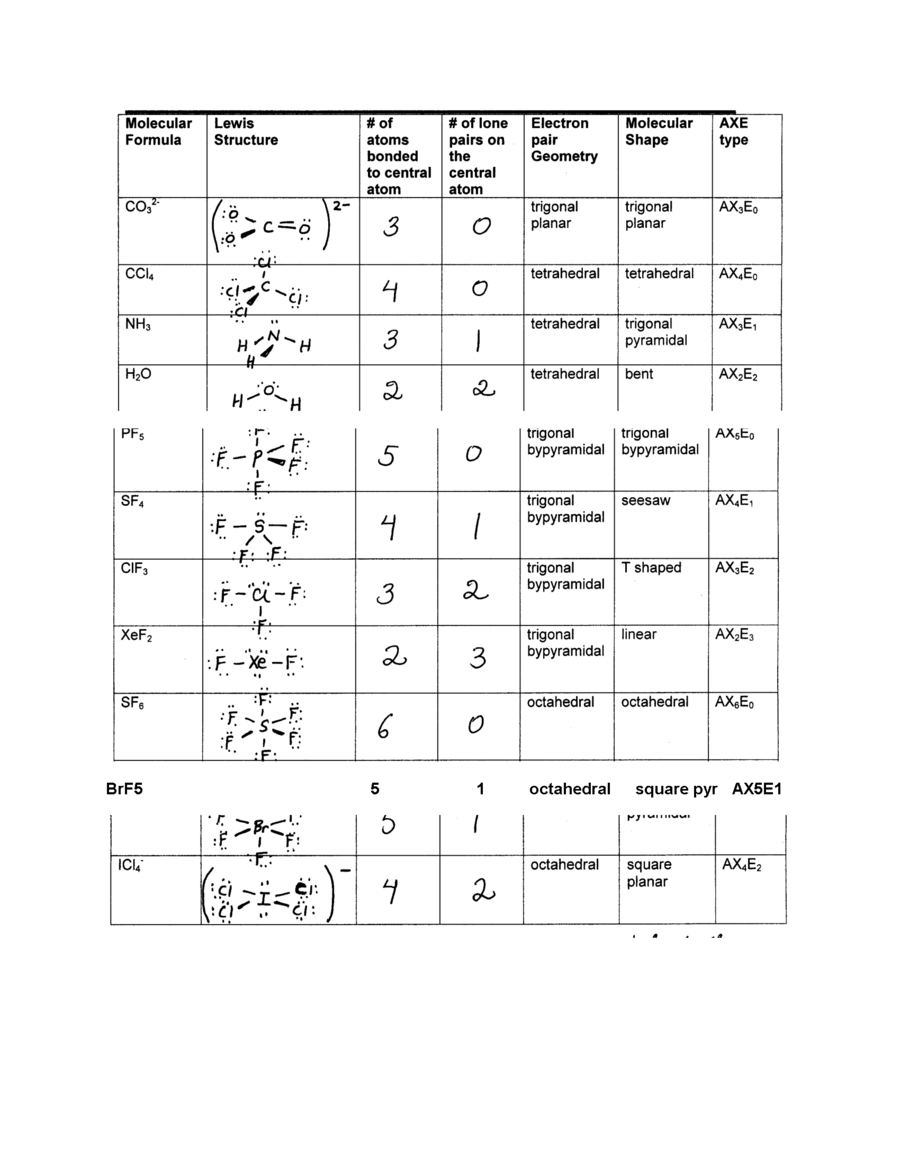
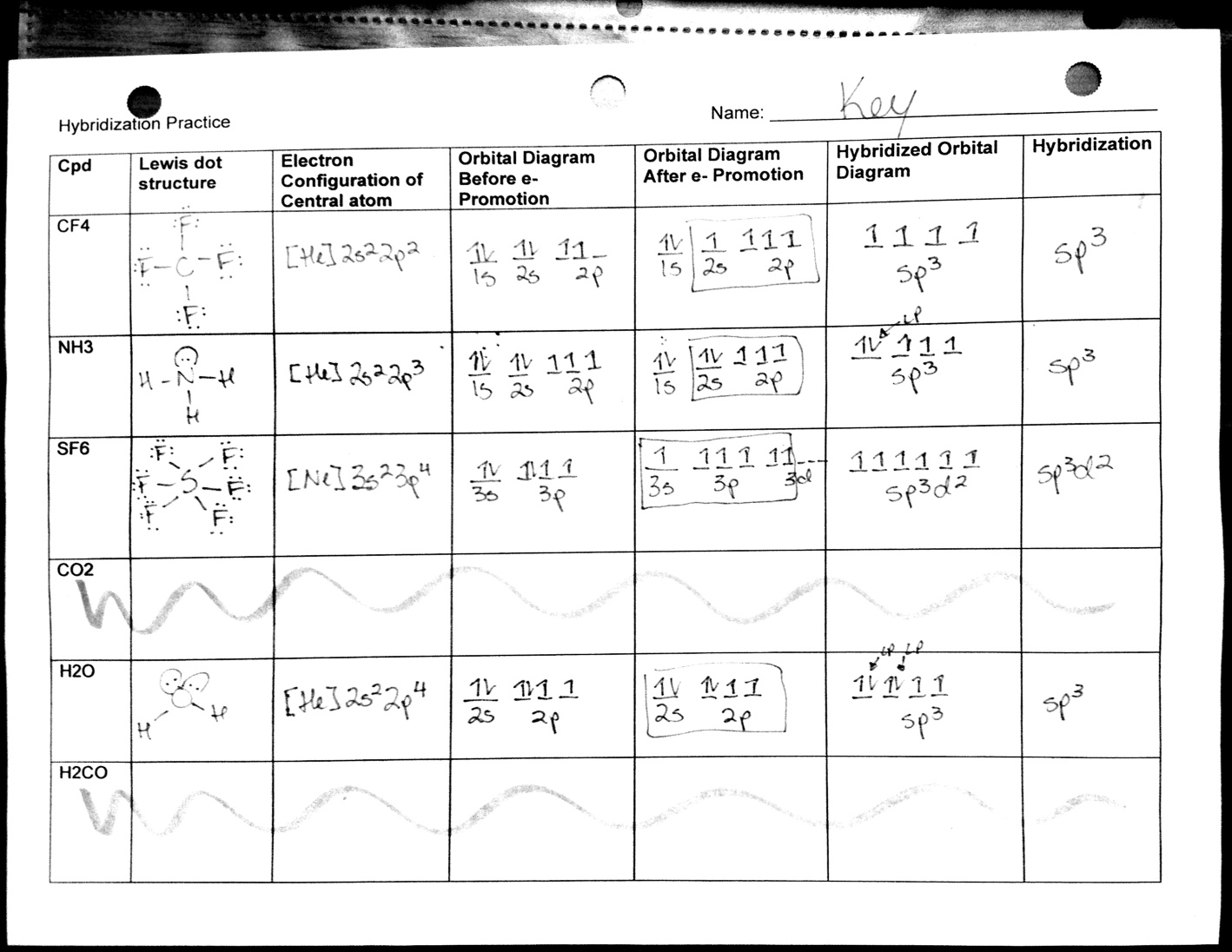
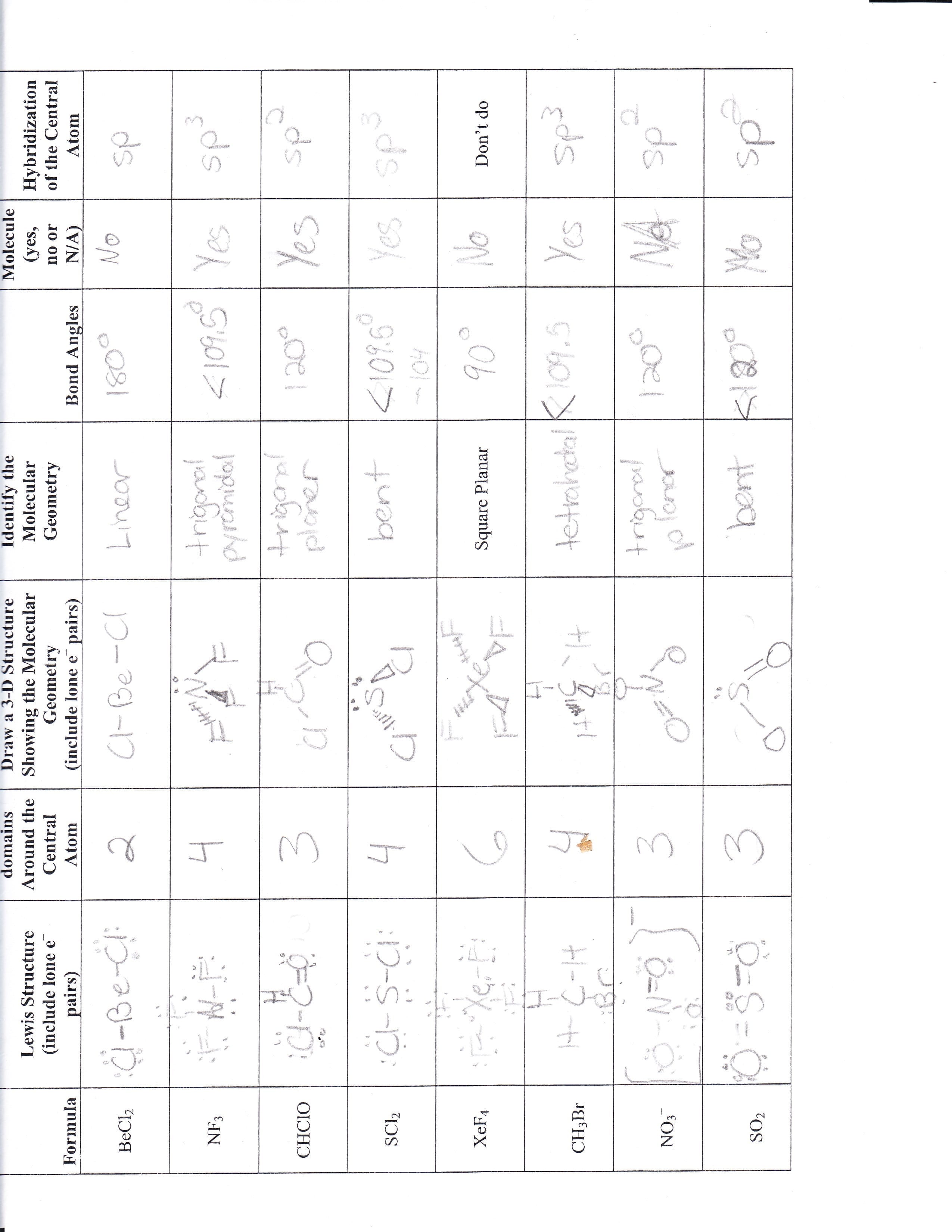
Molecular Shape And Polarity Worksheet Answers
Molecular Shape And Polarity Worksheet Answers - Bond dipoles are added up to determine the overall molecule dipole. H o h a) how many groups (atoms and lone pairs) surround the central oxygen? The molecular weight of \text {cocl}_2 cocl2 is 128.9\,\dfrac {\text {g}} {\text {mol}} 128.9 molg. A study of matter © 2004, gpb 10.18b 5. + 1 l.p.), and square planar (4 b.p. Bond dipoles point toward the more electronegative atom of the bond. Atoms assume a geometry dependent upon the electron pair geometry. Drawing lewis structures, molecular shape and polarity worksheet fill in the following table: What mass of \text {cocl}_2 cocl2 (in grams) is needed for the solution? (a) if the bond dipoles cancel, the molecule is nonpolar. If the polar bonds (dipoles + 1 l.p.), and square planar (4 b.p. Web shape of the molecule? • explain the relationship between bond dipoles and molecular dipole. Atoms assume a geometry dependent upon the electron pair geometry. Bond dipoles point toward the more electronegative atom of the bond. (see appendix b.) since all the pendant atoms are the same (b), the polarity must be the result of shape, and not composition. (b) if bond dipoles do not cancel, the molecule is polar. 4 b) what is the geometry of this molecule (look at atoms and lone pairs)?. Web in this workshop we will explore one of the models used in chemistry to predict molecular structure, the valence shell electron pair repulsion model, or vsepr. 4 b) what is the geometry of this molecule (look at atoms and lone pairs)? Draw this vsepr structure next to the lewis structure. Change solutes to compare different chemical compounds in water.. Web in this workshop we will explore one of the models used in chemistry to predict molecular structure, the valence shell electron pair repulsion model, or vsepr. A study of matter © 2004, gpb 10.18b 5. Bond dipoles point toward the more electronegative atom of the bond. Web molarity practice worksheet find the molarity (concentration) of the following solutions: +. 125 cm 3 of solution contains 3.5 moles of solute. Tetrahedron c) what is the shape of this molecule (look only at the atoms)? 5) this problem is also solved in the same way as #3 and #4. Web shape of the molecule? Drawing lewis structures, molecular shape and polarity worksheet fill in the following table: Molarity = mole/liters volume must be in liters! 4) this is done in the same method that you’d solve #3. 2) 3.0 moles of silver chloride is dissolved in enough water to make a 2.0 liter solution Draw this vsepr structure next to the lewis structure. 4 b) what is the geometry of this molecule (look at atoms and lone. (a) if the bond dipoles cancel, the molecule is nonpolar. • accurately predict and explain the bond dipoles and molecular dipoles of real molecules. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. Larger molecules do not have a single central atom, but are connected by a. G kno 3 = 0.175 mol kno. 5) this problem is also solved in the same way as #3 and #4. Draw this vsepr structure next to the lewis structure. 100% (1 rating) transcribed image text: 4 b) what is the geometry of this molecule (look at atoms and lone pairs)? Web in this workshop we will explore one of the models used in chemistry to predict molecular structure, the valence shell electron pair repulsion model, or vsepr. A study of matter © 2004, gpb 10.18b 5. Change solutes to compare different chemical compounds in water. 4) this is done in the same method that you’d solve #3. Learn about the. (b) if bond dipoles do not cancel, the molecule is polar. • accurately predict and explain the bond dipoles and molecular dipoles of real molecules. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. (see appendix b.) since all the pendant atoms are the same (b), the. 125 cm 3 of solution contains 3.5 moles of solute. Atoms assume a geometry dependent upon the electron pair geometry. 2) 3.0 moles of silver chloride is dissolved in enough water to make a 2.0 liter solution 4 b) what is the geometry of this molecule (look at atoms and lone pairs)? What is the molarity of the solution? Molecule lewis structure polarity molecular shape bond angles nh,br bci ch,cl2 no so h,0*. Change solutes to compare different chemical compounds in water. Larger molecules do not have a single central atom, but are connected by a chain of interior atoms that each possess a. Molarity = mole/liters volume must be in liters! The possible shapes for the formula ab 4 are tetrahedral (4 b.p.), irregular tetrahedron (4 b.p. Web what determines the concentration of a solution? Bond dipoles are added up to determine the overall molecule dipole. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. H o h a) how many groups (atoms and lone pairs) surround the central oxygen? Learn about the relationships between moles, liters, and molarity by adjusting the amount of solute and solution volume. 1 liter = 1000 mls 1) 2.5 moles of sodium chloride is dissolved to make 0.050 liters of solution. Bond dipoles point toward the more electronegative atom of the bond. • accurately predict and explain the bond dipoles and molecular dipoles of real molecules. (b) if bond dipoles do not cancel, the molecule is polar. Because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. Drawing lewis structures, molecular shape and polarity worksheet fill in the following table: (b) if bond dipoles do not cancel, the molecule is polar. But the key factor for determining the polarity of a molecule is its shape. A 0.674\,\text m 0.674m cobalt (ii) chloride ( \text {cocl}_2 cocl2) solution is prepared with a total volume of 0.0750\,\text {l} 0.0750l. Because you have 6.68 moles of li2so4 and 2.500 liters of water, the overall molarity of your solution is 2.67 m. Web the molecular geometry of a compound results from the relative orientation of its atoms based on its electron group geometry determining molecular geometries based on vsepr rules: Bond dipoles are added up to determine the overall molecule dipole. + 1 l.p.), and square planar (4 b.p. 100% (1 rating) transcribed image text: The molecular weight of \text {cocl}_2 cocl2 is 128.9\,\dfrac {\text {g}} {\text {mol}} 128.9 molg. • accurately predict and explain the bond dipoles and molecular dipoles of real molecules. What mass of \text {cocl}_2 cocl2 (in grams) is needed for the solution? Draw this vsepr structure next to the lewis structure. Web when a molecule or polyatomic ion has only one central atom, the molecular structure completely describes the shape of the molecule. Molarity = mole/liters volume must be in liters! 1 liter = 1000 mls 1) 2.5 moles of sodium chloride is dissolved to make 0.050 liters of solution.Polar And Nonpolar Bonds And Molecules Worksheet Answers Worksheet List
Worksheet Polarity Of Bonds Answers
6.5 Practice Worksheet B Polarity and Intermolecular Forces
Worksheet Polarity Of Bonds Answers Worksheet for Education in 2022
Molecular Geometry And Polarity Worksheets Answers
Molecular Geometry And Polarity Worksheets Answers
Molecular Geometry And Polarity Worksheets Answers —
molecular polarity worksheet answer key
Molecular Geometry And Polarity Worksheets Answers
Polarity And Electronegativity Worksheet Answers Coearth
What Is The Molarity Of The Solution?
Larger Molecules Do Not Have A Single Central Atom, But Are Connected By A Chain Of Interior Atoms That Each Possess A.
For The Molecule To Be Polar, It Must, Of Course, Have Polar Bonds.
2) 3.0 Moles Of Silver Chloride Is Dissolved In Enough Water To Make A 2.0 Liter Solution
Related Post: