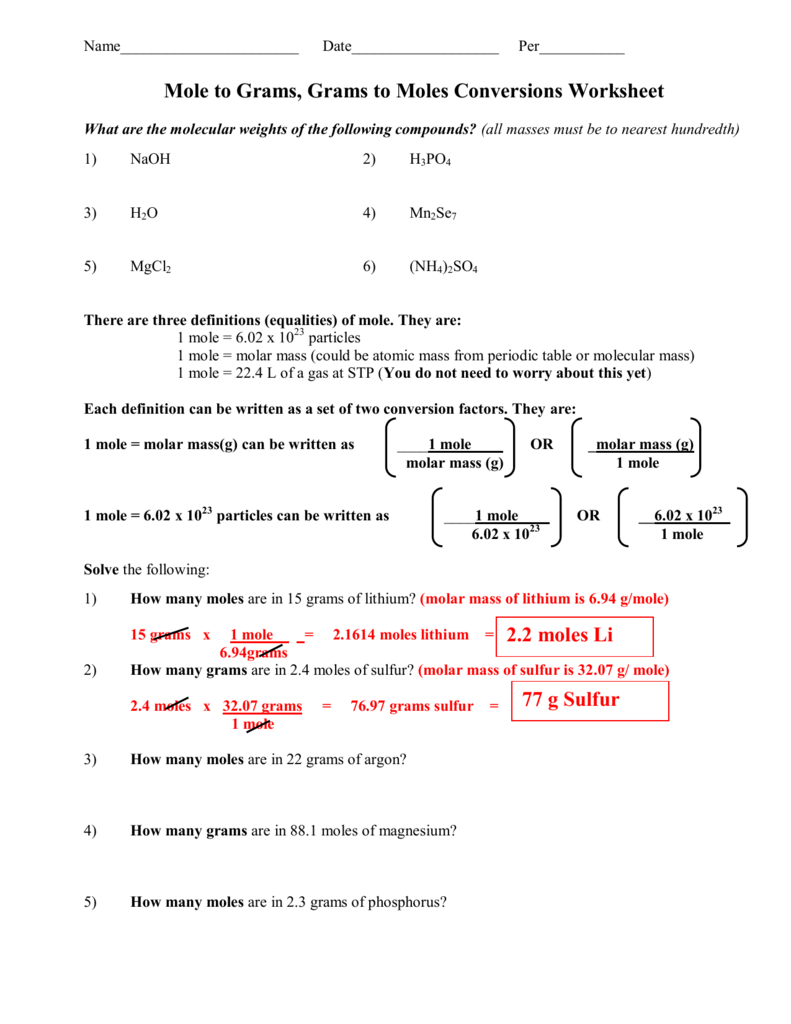
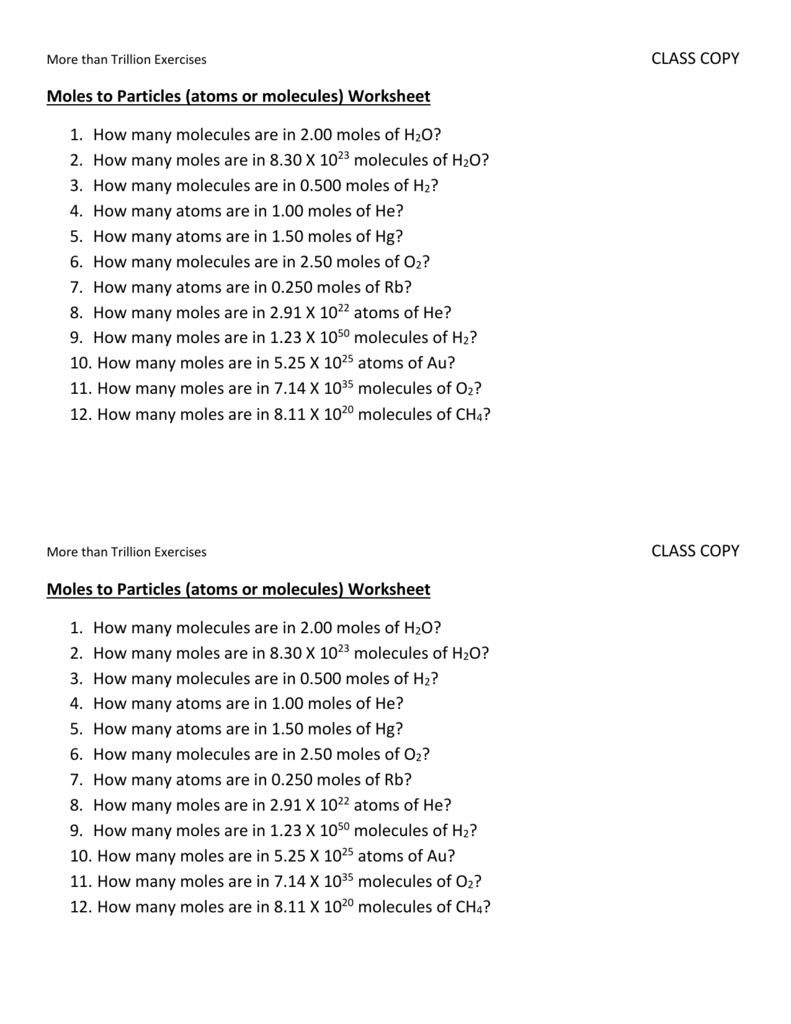
Moles To Particles Atoms Or Molecules Worksheet
Moles To Particles Atoms Or Molecules Worksheet - Web up to $3 cash back moles to particles (atoms or molecules) worksheet. Bundled set of 5 mole practice problem worksheets. How many molecules are in 2.00 moles of h2o? While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Web this worksheet is also available in a bundled product containing 5 different worksheets: Your students can solve mole calculations word problems and convert between moles,. Mole conversions work each of the following problems. Web moles to particles (atoms or molecules) worksheet. There are three mole equalities. Determine the number of moles of \(fe\) produced from 60.0 grams of. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Scientific notation is used.what is not included?there. 1 24 molecules h 2 o. Elements generally exist as the particles we call atoms. How many moles are in 8 x 10 23 molecules. How many atoms are in 6.2 moles of aluminum? How many moles are in 8 x 10 23 molecules. There are many applications for the mole unit in chemistry. Web moles to particles (atoms or molecules) worksheet. 1 mol = molar mass in g. 1 24 molecules h 2 o. Mole conversions work each of the following problems. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Convert 5.3 x 1025 molecules of co 2 to. How many atoms are in 6.2 moles of aluminum? Web practice converting particles to moles and moles to particles. There are many applications for the mole unit in chemistry. Web moles to particles (atoms or molecules) worksheet. Elements generally exist as the particles we call atoms. Your students can solve mole calculations word problems and convert between moles,. Mole conversions work each of the following problems. How many molecules are in 2.00 moles of h2o? How many moles are in 8.30 x 1023 molecules of. Elements generally exist as the particles we call atoms. Convert 5.3 x 1025 molecules of co 2 to. Web up to $3 cash back moles to particles (atoms or molecules) worksheet. 1 mol = 6.02 x 1023 particles. The words molecules , atoms and formula units are used. How many atoms are in 6.2 moles of aluminum? Convert 5.3 x 1025 molecules of co 2 to. Convert 5.3 x 1025 molecules of co 2 to. Your students can solve mole calculations word problems and convert between moles,. All answers and solutions are. 1 mol = molar mass in g. Web this worksheet is also available in a bundled product containing 5 different worksheets: The words molecules , atoms and formula units are used. 1 24 molecules h 2 o. Convert 5.3 x 1025 molecules of co 2 to. There are three mole equalities. How many molecules are in 2.00 moles of h2o? There are many applications for the mole unit in chemistry. Convert 5.3 x 1025 molecules of co 2 to. 1 24 molecules h 2 o. Web a mole, or 6.02 × 10 23 molecules, is a common unit used in chemistry to determine the amount of a substance. Web mole calculations worksheet color by number with chemistry mole conversions. Bundled set of 5 mole practice problem worksheets. Web up to $3 cash back moles to particles (atoms or molecules) worksheet. 1 24 molecules h 2 o. There are many applications for the mole unit in chemistry. Mole conversions work each of the following problems. All answers and solutions are. 1 mol = molar mass in g. The words molecules , atoms and formula units are used. Web moles to particles (atoms or molecules) worksheet. Convert 5.3 x 1025 molecules of co 2 to. Determine the number of moles of \(fe\) produced from 60.0 grams of. Convert 5.3 x 1025 molecules of co 2 to. Mole conversions work each of the following problems. There are three mole equalities. 1 24 molecules h 2 o. Web determine the number of moles of \(al_2o_3\) produced from 27.0 grams of \(al\). How many moles are in 8 x 10 23 molecules. Mole conversions work each of the following problems. Web practice converting particles to moles and moles to particles. 1 mol = 6.02 x 1023 particles. Web mole calculations worksheet color by number with chemistry mole conversions. How many moles are in 8.30 x 1023 molecules of. There are many applications for the mole unit in chemistry. While a dozen is only 12 particles a mole is a much larger number—6.02 x 1023 particles. Web “for every mole of \(c_3h_8(g)\), five moles of \(o_2(g)\) are required to produce three moles of \(co_2(g)\) and four moles of \(h_2o(l)\).” thus, we can. Bundled set of 5 mole practice problem worksheets. Mole conversions work each of the following problems. How many molecules are in 2 moles of h 2 o? How many molecules are in 2.00 moles of h2o? There are three mole equalities. Mole conversions work each of the following problems. All answers and solutions are. Web practice converting particles to moles and moles to particles. How many moles are in 8 x 10 23 molecules. Your students can solve mole calculations word problems and convert between moles,. Web a mole, or 6.02 × 10 23 molecules, is a common unit used in chemistry to determine the amount of a substance. Web determine the number of moles of \(al_2o_3\) produced from 27.0 grams of \(al\). 1 mol = molar mass in g. Convert 5.3 x 1025 molecules of co 2 to. How many atoms are in 6.2 moles of aluminum? Web this worksheet is also available in a bundled product containing 5 different worksheets:Figure of Converting Between Moles and Atoms Boundless mcat
️Mole Particle Practice Worksheet Free Download Goodimg.co
1 Mole Calculation Worksheet
MoleParticle Practice Worksheet
Molar Conversion Worksheet / Mole Calculation Worksheet The sum of
Moles to Particles (atoms or molecules) Worksheet
Moles To Molecules Conversion Worksheet With A Key
Moles, Molecules and Molecular Mass Worksheet for 9th 12th Grade
High School Chem mole conversions Math stuff Chemistry lessons
Moles Mass And Particles Worksheet Ivuyteq
How Many Atoms Are In 6.2 Moles Of Aluminum?
How Many Moles Are In 8.30 X 1023 Molecules Of.
While A Dozen Is Only 12 Particles A Mole Is A Much Larger Number—6.02 X 1023 Particles.
There Are Many Applications For The Mole Unit In Chemistry.
Related Post: