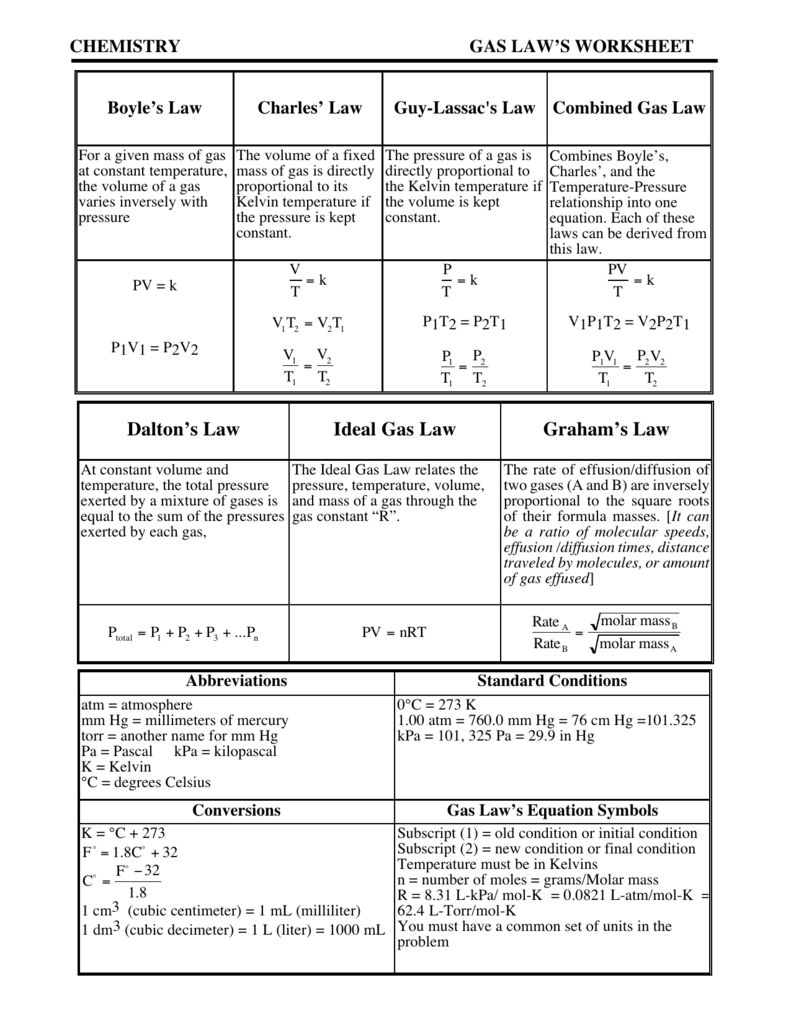
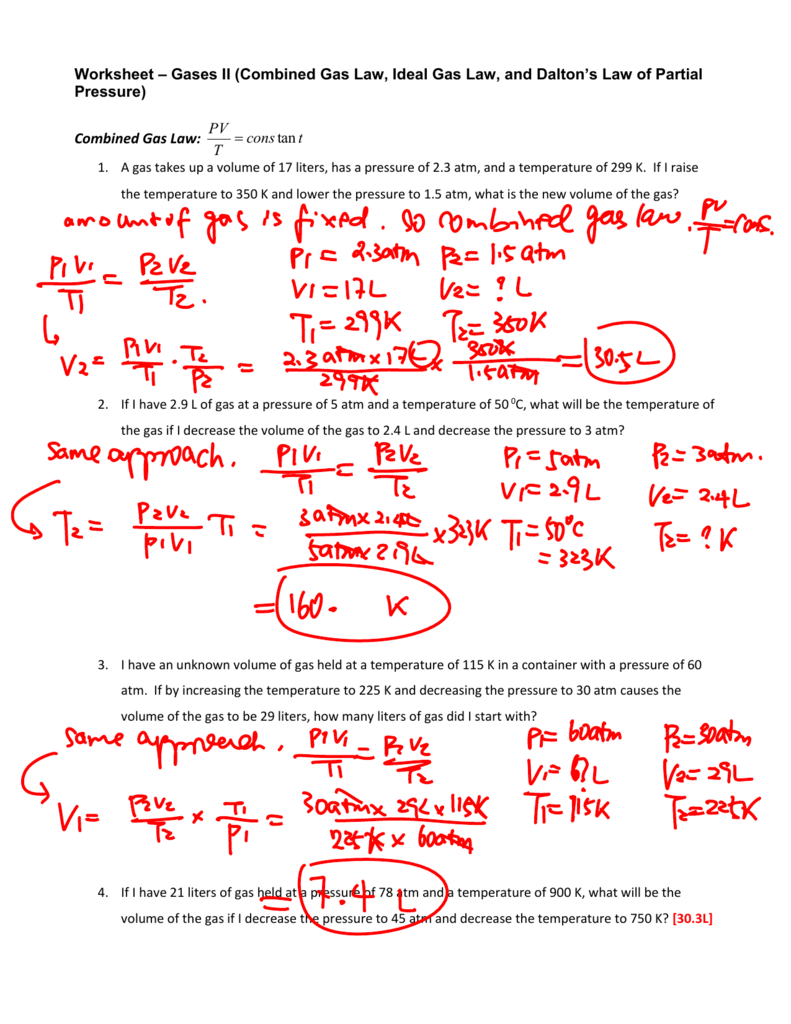
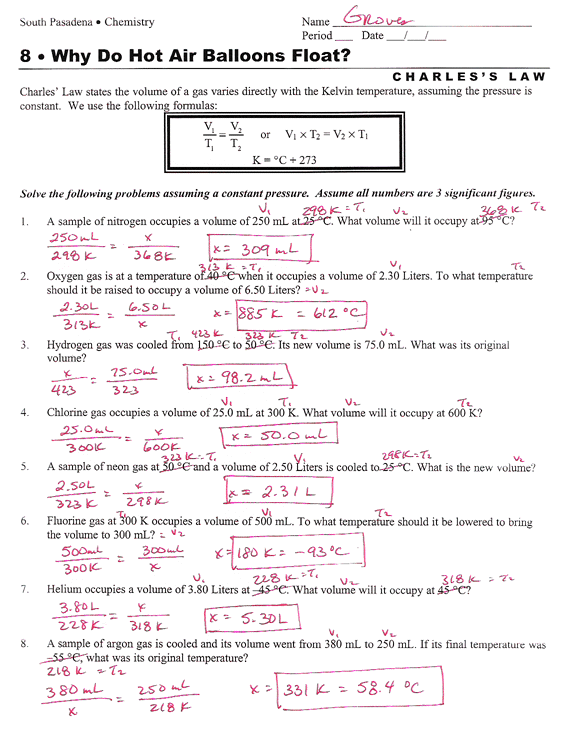
Partial Pressures Of Gas Chem Worksheet 14-6 Answer Key
Partial Pressures Of Gas Chem Worksheet 14-6 Answer Key - The total pressure of the mixture e. Web print dalton's law of partial pressures: Web as the pressure increases, the volume decreases. Although the problem does not explicitly state the pressure, it does tell you the balloon is at standard temperature and pressure. Web the partial pressure is the pressure the gas if the gas were in the same volume and temperature by itself. Web we refer to the pressure exerted by a specific gas in a mixture as its partial pressure. A gaseous mixture consists of 80 g of chlorine and 21 g of. In other words, mixtures of gases behave. What is the partial pressure of each gas in the mixture? What are the mole fractions of all the gases in the mixture? What are the mole fractions of all the gases in the mixture? Web dalton's law of partial pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure (p tot = p 1 + p 2 + p 3 +.) 1. = (0.045)(2.3 atm) = 0.10 atm p ! Web a. This worksheet describes the pressure due to a system of. A gaseous mixture consists of 80 g of chlorine and 21 g of. The mole fraction of each gas d. Web dalton's law of partial pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure (p tot = p 1. Web in terms of partial pressures: Web in a mixture of gases each individual gas contributes its own pressure, known as the partial pressure, to the total pressure. Web what is the partial pressure of each of the gases? Relating temperature, volume, and pressure; Web a sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2. Web until the gas pressure reaches 4.05 atm. Web print dalton's law of partial pressures: Calculating partial & total pressures worksheet 1. The partial pressure of a gas can be calculated using the ideal gas law, which we will cover. An increase in pressure forces the gas molecules closer. What is the partial pressure of each gas in the mixture? Web an increase in pressure forces the gas molecules closer. Web the oxygen has a partial pressure of 99 mm hg, nitrogen gas at 0.330 atm, and the total pressure is of 675 mm hg. Although the problem does not explicitly state the pressure, it does tell you the. Web an increase in pressure forces the gas molecules closer. Although the problem does not explicitly state the pressure, it does tell you the balloon is at standard temperature and pressure. = (0.195)(2.3 atm) = 0.45 atm check your answer by. Web college chemistry help» solutions, states of matter, and thermochemistry» gases» partial pressure. The pressure of each gas is. Web print dalton's law of partial pressures: Web what is the partial pressure of each of the gases? Web we refer to the pressure exerted by a specific gas in a mixture as its partial pressure. Web dalton’s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the individual. = (0.195)(2.3 atm) = 0.45 atm check your answer by. Relating temperature, volume, and pressure; Web in terms of partial pressures: The partial pressure of a gas can be calculated using the ideal gas law, which we will cover. Web in a mixture of gases each individual gas contributes its own pressure, known as the partial pressure, to the total. Web a sample of gas isolated from unrefined petroleum contains 90.0% ch 4, 8.9% c 2 h 6, and 1.1% c 3 h 8 at a total pressure of 307.2 kpa. Web as the pressure increases, the volume decreases. Ideal gas law fact and conceptual questions; The volume of the gas particles. The pressure of each gas is determined by. The pressure of each gas is determined by the. Although the problem does not explicitly state the pressure, it does tell you the balloon is at standard temperature and pressure. Relating temperature, volume, and pressure; = (0.76)(2.3 atm) = 1.75 atm p #! This worksheet describes the pressure due to a system of. Web dalton’s law of partial pressures states that the total pressure of a mixture of gases is equal to the sum of the individual pressures. = (0.195)(2.3 atm) = 0.45 atm check your answer by. What are the mole fractions of all the gases in the mixture? = (0.045)(2.3 atm) = 0.10 atm p ! Web an increase in pressure forces the gas molecules closer. = (0.76)(2.3 atm) = 1.75 atm p #! Although the problem does not explicitly state the pressure, it does tell you the balloon is at standard temperature and pressure. Web the oxygen has a partial pressure of 99 mm hg, nitrogen gas at 0.330 atm, and the total pressure is of 675 mm hg. Web in terms of partial pressures: The pressure of each gas is determined by the. Keq = kp = (the subscript “p” stands for “pressure”.) the numerical value of the two equilibrium constants above will be different. Relating temperature, volume, and pressure; The volume of the gas particles. Web what is the total pressure of the gas? What is the partial pressure of each gas in the. Web until the gas pressure reaches 4.05 atm. In other words, mixtures of gases behave. Web the partial pressure is the pressure the gas if the gas were in the same volume and temperature by itself. The partial pressure of each gas 4. An increase in pressure forces the gas molecules closer. The total pressure of the mixture e. = (0.045)(2.3 atm) = 0.10 atm p ! This worksheet describes the pressure due to a system of. The partial pressure of a gas can be calculated using the ideal gas law, which we will cover. Web an increase in pressure forces the gas molecules closer. Web dalton's law of partial pressures states that the sums of the pressures of each gas in the mixture add to give the total pressure (p tot = p 1 + p 2 + p 3 +.) 1. The mole fraction of each gas d. Web what is the total pressure of the gas? Web as the pressure increases, the volume decreases. The partial pressure of each gas 4. What is the partial pressure of each gas in the mixture? Web in terms of partial pressures: In other words, mixtures of gases behave. Helium is then pumped in until the total pressure is 20.00 atm. Web the partial pressure is the pressure the gas if the gas were in the same volume and temperature by itself. Calculating partial & total pressures worksheet 1.Solved Dalton 's Law of Partial Pressures Worksheet 1. Blast
The Combined Gas Law Relates Which Of The Following slidesharetrick
Worksheet Gas Laws II Answers
Gas Stoichiometry Worksheet Answer Key
Gas Laws Worksheet III Answer Key 1112 Gases Mole (Unit)
Partial Pressures Of Gas Chem Worksheet 146 Answer Key
Partial Pressures of Gas Name Chem Worksheet 146 JTE35633/worksheets
Gas Laws Worksheet Answer Key Gases Litre
Gas Law Gizmo Sheet Answers / Student Exploration Boyle S Law And
Gen Chem Page Worksheet Template Tips And Reviews
Web In A Mixture Of Gases Each Individual Gas Contributes Its Own Pressure, Known As The Partial Pressure, To The Total Pressure.
Ideal Gas Law Fact And Conceptual Questions;
What Would Be The Partial Pressure Of Each Of The Gases In A Container At 50 °C In Which There Is 0.20 Mole N2 And 0.10 Mole Co2 At A Total Pressure Of 101.3 Kpa?
Keq = Kp = (The Subscript “P” Stands For “Pressure”.) The Numerical Value Of The Two Equilibrium Constants Above Will Be Different.
Related Post: