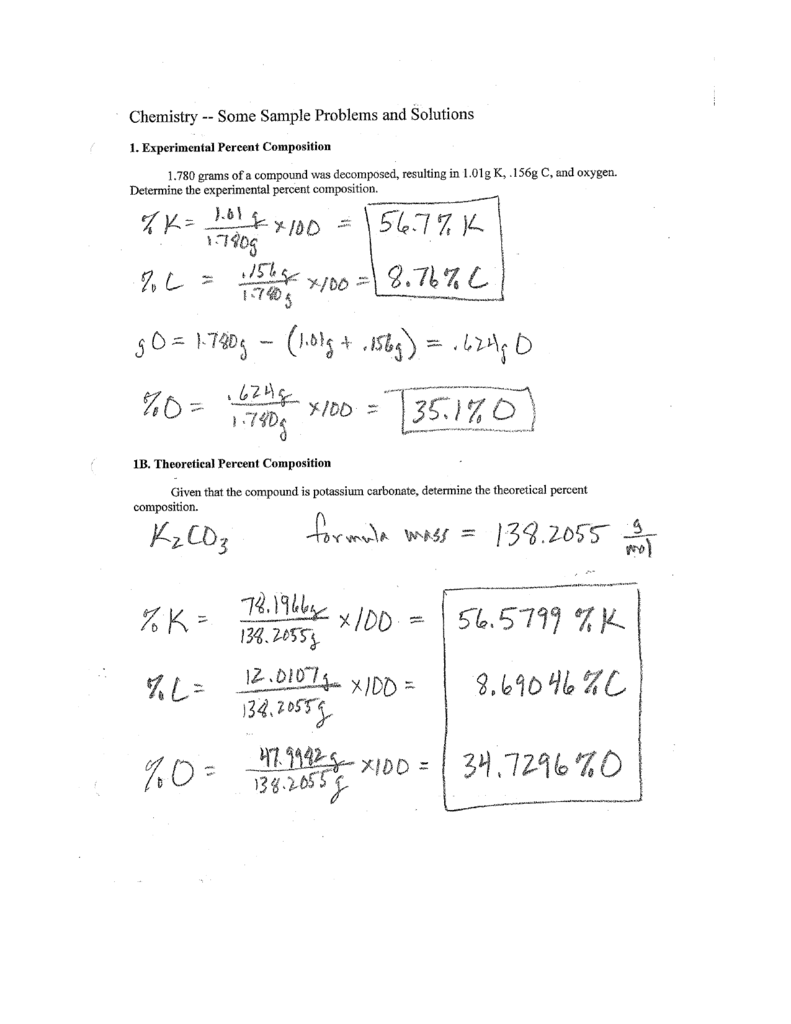
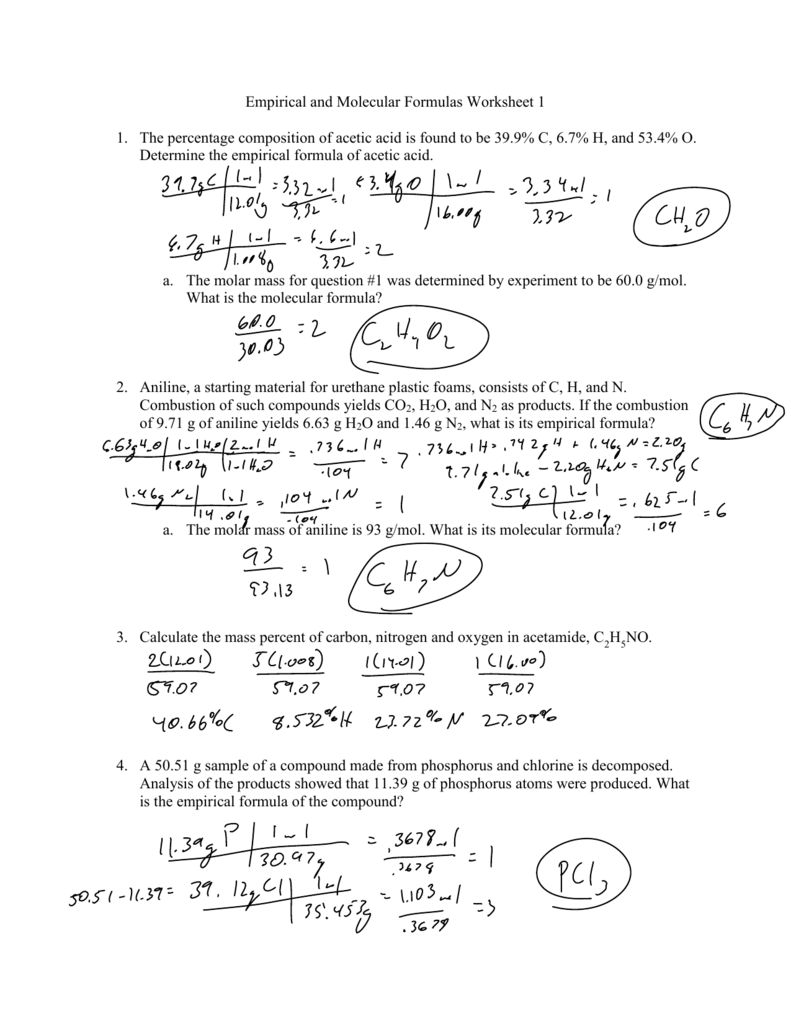
Percent Composition Empirical And Molecular Formulas Worksheet
Percent Composition Empirical And Molecular Formulas Worksheet - The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Join to access all included materials. Empirical formulas work each of the following problems. What is the empirical formula? Calculate the percent composition of a substance from its chemical formula or experimental data. Show your work, and always include units where needed. Web determining percent composition from molecular or empirical formulas. What is the percent composition of so 2? Web a component of protein called serine has an approximate molar mass of 100 g/mole. Web write the empirical formula for the following compounds. Show your work, and always include units where needed. Join to access all included materials. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % c7h7no. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % 15. Web the compound benzamide has the following percent composition. Web the compound benzamide has the following percent composition. (assume percentages given in the problems are grams) step 1:. If the percent composition is as follows, what is the empirical and molecular formula of. Identify empirical and molecular formulas. Web a component of protein called serine has an approximate molar mass of 100 g/mole. What is the percent composition of calcium. Calculate the percent by mass. What is the percent composition of so 2? Web this ws 4.5 percent composition and empirical formula worksheet also includes: Web a sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. (assume percentages given in the problems are grams) step 1:. What is the percent composition of so 2? S = _____ % o = _____ % 2. Calculate the percent composition of a substance from its chemical formula or experimental data. 1) c6h6 2) c8h18 3) wo2 4) c2h6o2 5) x39y13 6) a compound with an empirical formula of c2oh4. Web determining percent composition from molecular or empirical formulas. Percentage composition and empirical & molecular formula. What is the empirical formula? Web this is a package of two worksheets. What is the empirical formula? Identify empirical and molecular formulas. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. Web a component of protein called serine has an approximate molar mass of 100 g/mole. Calculate the percent by mass. Web percent composition and molecular formula worksheet 1) what’s the empirical formula. What is the percent composition of so 2? This unit is meant to cover the basics of. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. Web the compound benzamide has the following percent composition. Calculate the percent by mass. Web a component of protein called serine has an approximate molar mass of 100 g/mole. Web answers to worksheet #8 empirical formulas to calculate empirical formulas, follow the steps outlined below: C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % c7h7no. Web empirical formulas, and molecular formulas 1. What is the percent composition of calcium. Web the compound benzamide has the following percent composition. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of stoichiometry! Web empirical formulas, and molecular formulas 1. The first worksheet gives your students practice with finding the percent composition of a compound from a given formula, finding percent. Web percent composition and. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % c7h7no. Web a sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. Join to access all included materials. What is the empirical formula? Web this ws 4.5 percent composition and empirical formula worksheet also includes: Show your work, and always include units where needed. What is the percent composition of so 2? Web empirical formulas, and molecular formulas 1. Calculate the percent by mass. A compound is found to contain 63.52 % iron and 36.48 % sulfur. Find the percent composition of each element in the unknown compound. Web this is a package of two worksheets. S = _____ % o = _____ % 2. Web percent composition and molecular formula worksheet 1) what’s the empirical formula of a molecule containing 65.5% carbon, 5.5% hydrogen, and 29.0% oxygen? Web the compound benzamide has the following percent composition. Web determining percent composition from molecular or empirical formulas. The compound benzamide has the following percent composition. (assume percentages given in the problems are grams) step 1:. What is the empirical formula? C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % c7h7no. 1) c6h6 2) c8h18 3) wo2 4) c2h6o2 5) x39y13 6) a compound with an empirical formula of c2oh4 and a molar. Calculate the percent composition of a substance from its chemical formula or experimental data. Web a component of protein called serine has an approximate molar mass of 100 g/mole. C = 69.40 % h= 5.825 % o = 13.21 % n= 11.57 % 15. Percentage composition and empirical & molecular formula. Web this is a package of two worksheets. Web a sample of an unknown substance is found to have 49.37 g c, 4.14 g h and 21.86 g o. This unit is meant to cover the basics of. Web guided worksheet that reviews determining percent composition of a compound, finding hydrate formulas based on mass from a dehydration reaction, using percent. What is the percent composition of calcium. S = _____ % o = _____ % 2. Web empirical formulas, and molecular formulas 1. Web these high school chemistry worksheets are full of pictures, diagrams, and deeper questions covering all aspects of stoichiometry! Web this ws 4.5 percent composition and empirical formula worksheet also includes: A compound is found to contain 63.52 % iron and 36.48 % sulfur. If the percent composition is as follows, what is the empirical and molecular formula of. Percent composition is also useful for evaluating the relative abundance of a given element in. 1) c6h6 2) c8h18 3) wo2 4) c2h6o2 5) x39y13 6) a compound with an empirical formula of c2oh4 and a molar. Join to access all included materials. Calculate the percent composition of a substance from its chemical formula or experimental data. Web determining percent composition from molecular or empirical formulas.Percentage Composition and Empirical & Molecular Formula Worksheet for
Molecular Mass And Percent Composition Worksheet Answers Worksheet List
12 Best Images of Empirical Formula Worksheet With Answers Molecular
Empirical And Molecular Formula Worksheet —
Empirical Formula From Percent Composition Practice Problems
30 Awesome Molar Mass Chem Worksheet 11 2 Answer Key Worksheet and
Empirical and Molecular Formulas Worksheet 1 1. The percentage
empirical and molecular formula worksheet
comp, Emp & molec Formula MS MCLARTY'S CLASSES
Empirical Formula Worksheet Answers With Work Organicfer
Find The Percent Composition Of Each Element In The Unknown Compound.
Identify Empirical And Molecular Formulas.
Calculate The Percent By Mass.
(Assume Percentages Given In The Problems Are Grams) Step 1:.
Related Post: