Percent Yield Worksheet Answers
Percent Yield Worksheet Answers - Web what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the other reactants? Web up to 24% cash back (18.5 / 17.2) x 100% = 108% yield c) is the answer from problem #3 reasonable? 2hgo (s) 2hg (l) + o2 (g) in a certain reaction, 4.37 g of hgo is decomposed, producing 3.21 g of hg. Web the percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: Add to my workbooks (3) Calculate the percent yield of a reaction that produced 0.350 mol hcl if the. Yield is 17 g nh. Web limiting reagent worksheet #1 1. Web actual yield is 14 grams, what is the percent yield? Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. Hw 6.10 electron configs 2; 3, % yield = 82.3%. What percentage yield of iodine was produced. Web percent yield = 3.6: Add to my workbooks (3) Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. When carbon disulfide burns in the. Web the percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: I got a percent yield of 75% how many. 3 + 3 fe 2 sb + 3. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. Web what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the other reactants? 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your. Web limiting reagent worksheet #1 1. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. Web percent yield = 3.6: 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Web what is the percent yield of i 2 if the actual grams produced is. Web the percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: Web calculate the percent yield. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. 0/0 yield =. Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. Add to my workbooks (3) What percentage yield of iodine was produced. 2) according to the following equation, calculate the percentage yield if 550.0 g of toluene (c7h8 )added to an excess. 3, % yield = 82.3%. Web limiting reagent worksheet #1 1. Web what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the other reactants? The percent yield is calculated as. Web up to 24% cash back (18.5 / 17.2) x 100% = 108% yield c) is. I got a percent yield of 75% how many. Add to my workbooks (3) Web limiting reagent worksheet #1 1. 2) according to the following equation, calculate the percentage yield if 550.0 g of toluene (c7h8 )added to an excess. Web based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. 4 x 100 percent yield = 0.853 x 100 = 85.3% correct answer: Web actual yield is 14 grams, what is the percent yield? Add to my workbooks (3) What is the theoretical yield if 45.6 g of benzene react? Calculate the percent yield of a reaction that produced 0.350 mol hcl if the. 3 + 3 fe 2 sb + 3. Web what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the other reactants? Web actual yield is 14 grams, what is the percent yield? 4 x 100 percent yield = 0.853 x 100. Any yield over 100% is a violation of the law of conservation of. Web based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. What percentage yield of iodine was produced. C 3h 8 + 5 o 2 3 co 2 + 4 h 2o a. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. 2hgo (s) 2hg (l) + o2 (g) in a certain reaction, 4.37 g of hgo is decomposed, producing 3.21 g of hg. Web the percent yield of the reaction is the ratio of the actual over the theoretical yield, so rearranging this equation we can calculate the actual yield: 3 + 3 mg(oh) 2. Web what is my percent yield? Web calculate the percent yield. Hw 6.10 electron configs 2; Yield is 17 g nh. Calculate the percent yield by dividing the actual yield. When carbon disulfide burns in the. Calculate the percent yield of a reaction that produced 0.350 mol hcl if the. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. 4 x 100 percent yield = 0.853 x 100 = 85.3% correct answer: 3, % yield = 82.3%. I got a percent yield of 75% how many. Web based on the number of moles of the limiting reactant, use mole ratios to determine the theoretical yield. When carbon disulfide burns in the. If the actual yield is 63.7 g of chlorobenzene, calculate the percent yield. What is the percent yield of the. Web percent yield = 3.6: The percent yield is calculated as. Calculate the percent yield by dividing the actual yield. Web what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the other reactants? Web up to 24% cash back (18.5 / 17.2) x 100% = 108% yield c) is the answer from problem #3 reasonable? 2) according to the following equation, calculate the percentage yield if 550.0 g of toluene (c7h8 )added to an excess. Calculate the percent yield of a reaction that produced 0.350 mol hcl if the. C 3h 8 + 5 o 2 3 co 2 + 4 h 2o a. 3, % yield = 82.3%. Worksheets are percent yield work, work percent yield name, percent yield and limiting reagents, chem1001 work 5. What percentage yield of iodine was produced. 3 + 3 mg(oh) 2.W.LimitingReagentsandPercentYield.HW1.ANSWERKEY
limiting reagent practice worksheet
Theoretical and Percent Yield Worksheet Answers
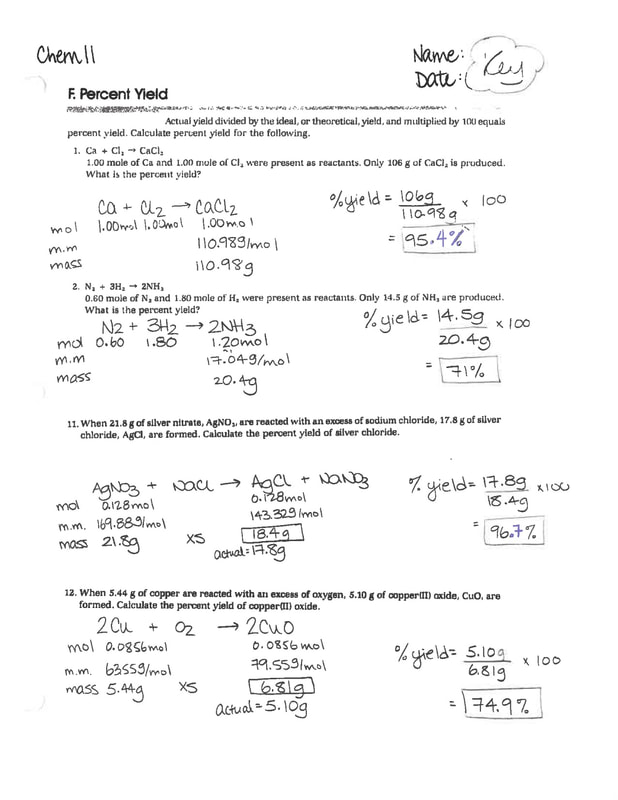
MS MCLARTY'S CLASSES Chem 11
Solved Name Stoichiometry Percent Yield Worksheet SHOW ALL
Theoretical and Percent Yield Worksheet Answers
Limiting Reactant And Percent Yield Worksheet Answer Key —
14 Stoichiometry Worksheet 2 Answer Key /
Limiting Reagent Worksheet
Limiting Reactant And Percent Yield Worksheet Answer Key —
Hw 6.10 Electron Configs 2;
Web The Actual Yield Of A Reaction Is Typically Reported As A Percent Yield, Or The Percentage Of The Theoretical Yield That Was Actually Obtained.
Web What Is My Percent Yield?
Web Actual Yield Is 14 Grams, What Is The Percent Yield?
Related Post: