Periodic Trends Ionization Energy Worksheet Answers
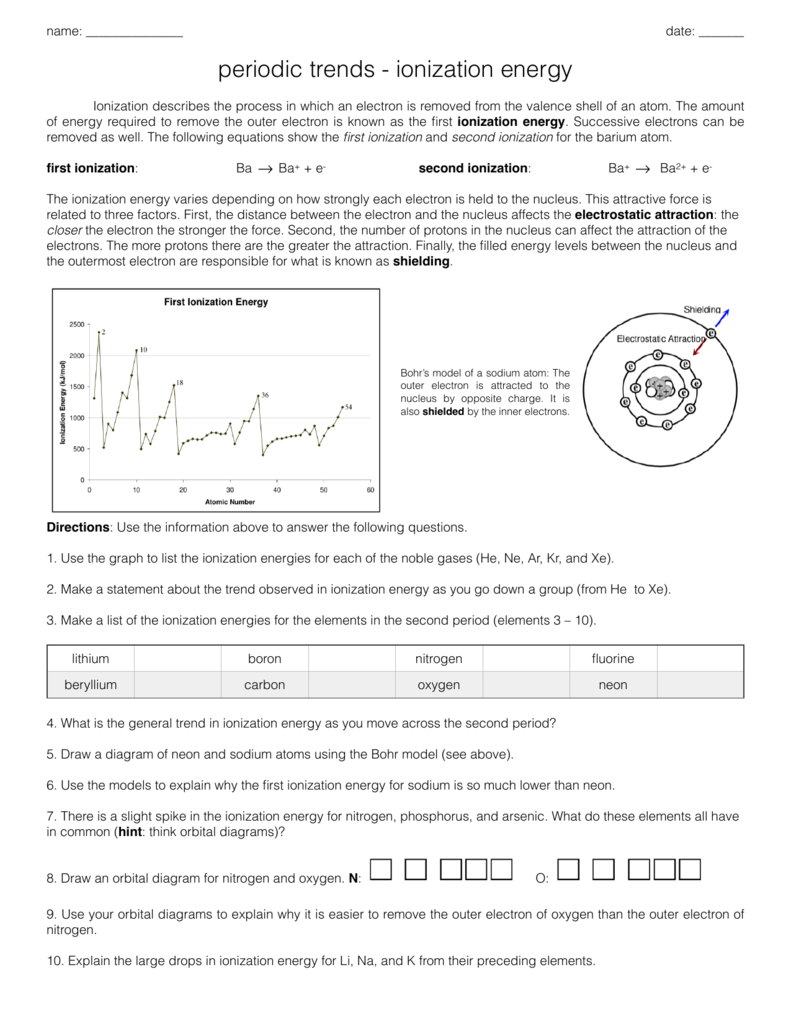
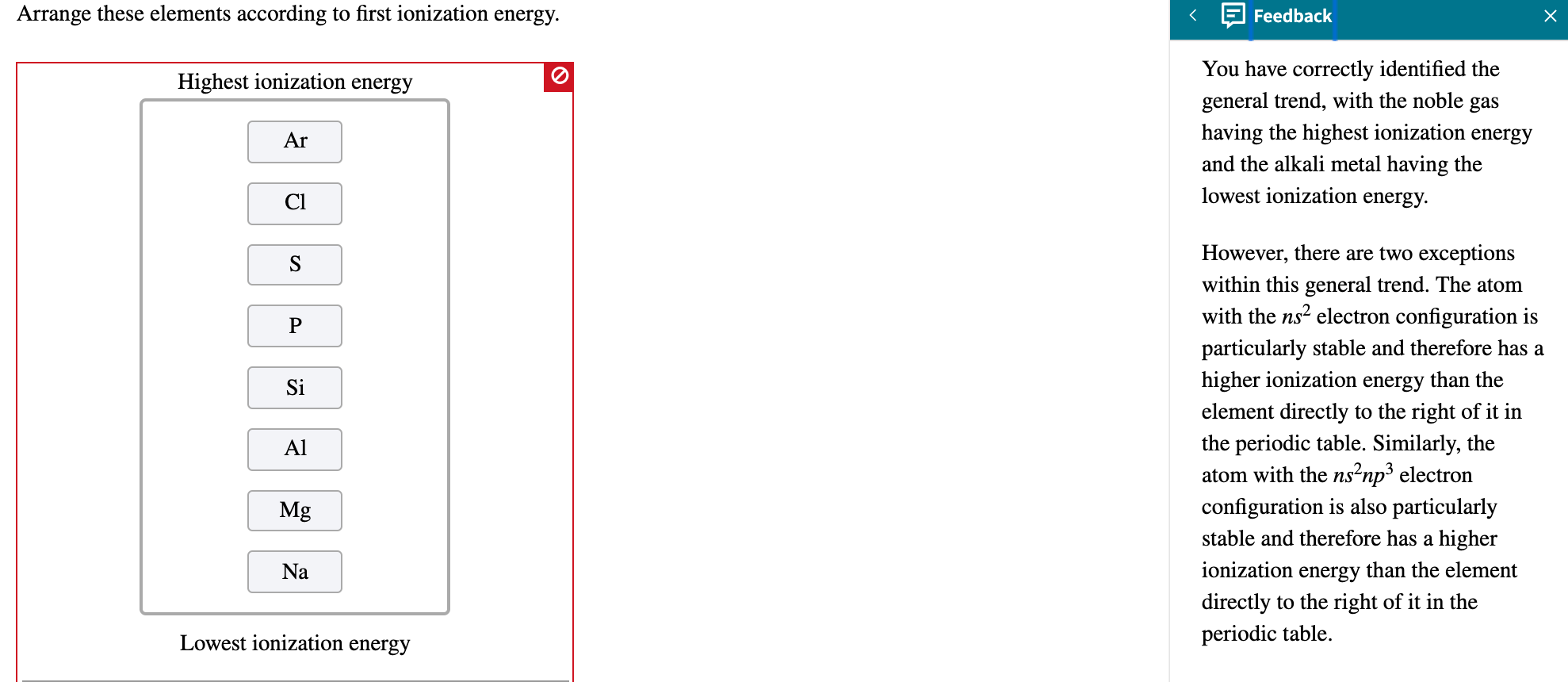
Periodic Trends Ionization Energy Worksheet Answers - Vocabulary:atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. Web what is the trend for ionization energy as one goes across period 3? What is the ionization energy of an. Web another property of atoms is called the ionization energy. Web use the chart above to answer the following questions. Ionization energy for each of the following sets of atoms, rank them from lowest to highest ionization energy. This bundle of 5 homework assignments includes questions that reviews. Use the graph to list the ionization energies for the noble gases (he, ne, ar, kr, and xe). Na, li, be (e) largest atom: Rank the following elements by increasing atomic radius: Web (a) highest first ionization energy: Web 8) define ionization energy. As, sn, s (d) smallest atom: What is the atomic number? O, ne ca, sr k, cr br, sb in, sn 4. Here is a table of the first ionization. Increases down group because energy level shells are added what trend in. Based on position in the periodic table, which element of the following pairs has the higher first ionization energy? 9) write the chemical equation that corresponds to the ionization energy of iodine. Web 8) define ionization energy. 9) write the chemical equation that corresponds to the ionization energy of iodine. O, ne ca, sr k, cr br, sb in, sn 4. Based on position in the periodic table, which element of the following pairs has the higher first ionization energy? Web 8) define ionization energy. 10) on the periodic table below, use arrows to show the. Choose the element with the greatest first ionization energy: Web what trend in ionization energy occurs across a period on the periodic table? Ionization energy for each of the following sets of atoms, rank them from lowest to highest ionization energy. Vocabulary:atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. O, ne ca, sr k, cr br, sb. Vocabulary:atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. O, ne ca, sr k, cr br, sb in, sn 4. Web what trend in atomic radius occurs down a group on the periodic table?what causes this trend? As, sn, s (d) smallest atom: Increases down group because energy level shells are added what trend in. Web what is the trend for ionization energy as one goes across period 3? Circle the atom in each pair that has the largest atomic radius. Web 8) define ionization energy. Use the graph to list the ionization energies for the noble gases (he, ne, ar, kr, and xe). C, n, si (b) largest radius: Use your notes to answer the following questions. As, sn, s (d) smallest atom: Web what is the trend for ionization energy as one goes across period 3? Web (a) highest first ionization energy: Rank the following elements by increasing atomic radius: This bundle of 5 homework assignments includes questions that reviews. It is the energy required to remove an electron from an atom in the gas phase. Web another property of atoms is called the ionization energy. 9) write the chemical equation that corresponds to the ionization energy of iodine. C, n, si (b) largest radius: Here is a table of the first ionization. It is the energy required to remove an electron from an atom in the gas phase. Web 8) define ionization energy. Web what is the trend for ionization energy as one goes across period 3? Increases down group because energy level shells are added what trend in. What is the atomic number? The number of protons found in an element. Web another property of atoms is called the ionization energy. The trend for ionization energy as you go across a period is that ionization energy increases b/c of. Web what is the trend for ionization energy as one goes across period 3? This bundle of 5 homework assignments includes questions that reviews. Use the graph to list the ionization energies for the noble gases (he, ne, ar, kr, and xe). Web use the chart above to answer the following questions. Web 8) define ionization energy. The trend for ionization energy as you go across a period is that ionization energy increases b/c of. Circle the atom in each pair that has the largest atomic radius. As, sn, s (d) smallest atom: Increases down group because energy level shells are added what trend in. What is the ionization energy of an. Web another property of atoms is called the ionization energy. Ionization energy for each of the following sets of atoms, rank them from lowest to highest ionization energy. It is the energy required to remove an electron from an atom in the gas phase. 9) write the chemical equation that corresponds to the ionization energy of iodine. C, n, si (b) largest radius: Web what trend in atomic radius occurs down a group on the periodic table?what causes this trend? Vocabulary:atomic radius, electron affinity, electron cloud, energy level, group, ion, ionization. What is the atomic number? The number of protons found in an element. Based on position in the periodic table, which element of the following pairs has the higher first ionization energy? Web what is the trend for ionization energy as one goes across period 3? Increases down group because energy level shells are added what trend in. C, n, si (b) largest radius: The number of protons found in an element. Web what trend in atomic radius occurs down a group on the periodic table?what causes this trend? Circle the atom in each pair that has the largest atomic radius. This bundle of 5 homework assignments includes questions that reviews. 9) write the chemical equation that corresponds to the ionization energy of iodine. What is the ionization energy of an. Web what trend in ionization energy occurs across a period on the periodic table? Use your notes to answer the following questions. The trend for ionization energy as you go across a period is that ionization energy increases b/c of. Na, li, be (e) largest atom: Based on position in the periodic table, which element of the following pairs has the higher first ionization energy? Web 8) define ionization energy. Web use the chart above to answer the following questions. Web another property of atoms is called the ionization energy.35 Chemistry Chapter 6 The Periodic Table Worksheet Answers support
Periodic Trends Ionization Energy YouTube
Trends in the Periodic Table 9th 12th Grade Worksheet Chemistry
ionization energy worksheet
Periodic Table Trends Worksheet Answers Chemistry A Study Of Matter
First Ionization Energy Trend Periodic Table Images Amashusho
Periodic Trends Worksheet Answer Key
Periodic Trends Practice Answer Key
Practice Ionization Energy & Orbitals Worksheet printable pdf download
Chemistry Ionization Energy Worksheet Answers Free Worksheets Samples
As, Sn, S (D) Smallest Atom:
Choose The Element With The Greatest First Ionization Energy:
What Is The Atomic Number?
O, Ne Ca, Sr K, Cr Br, Sb In, Sn 4.
Related Post: