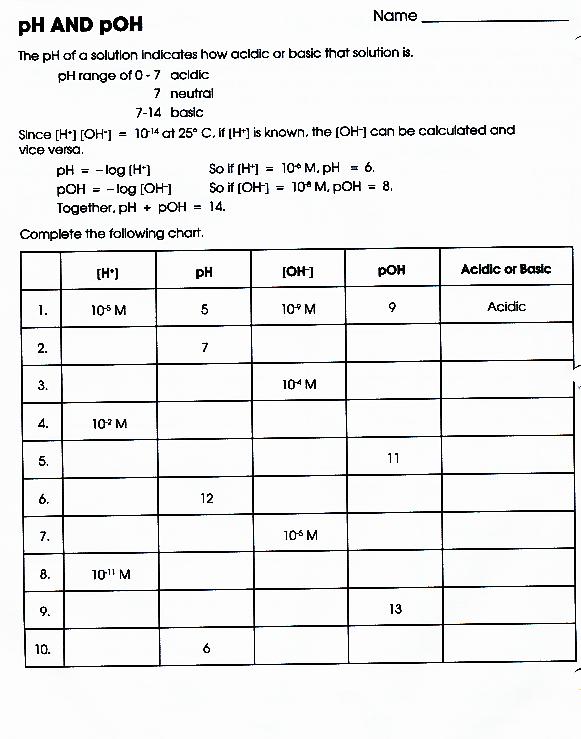
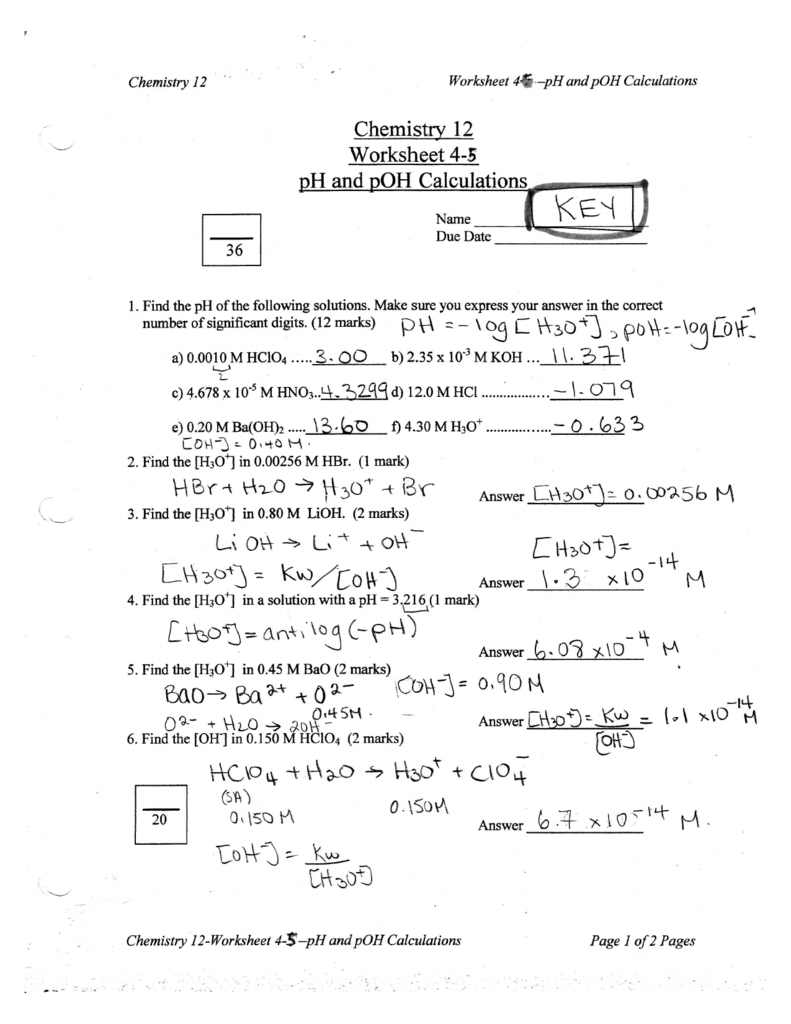
Ph And Poh Calculations Worksheet 2
Ph And Poh Calculations Worksheet 2 - Fill in the missing information in the table below. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. Web up to 6% cash back we can then derive the poh from the ph value. Ph and poh calculations worksheet #2. Web ph and poh calculations. A 0.023 m solution of hydrochloric acid. Names _____ date _____ class _____ find the [h 3 o+]. Web find the ph and the poh of the solution. Find the ph and the poh of the solution. Calculate the ph and the poh of each of the following solutions at 25 °c for. Names _____ date _____ class _____ find the [h 3 o+]. Find the ph and the poh of the solution. Names __________________________________________ date ____________ class _______. A 0.023 m solution of hydrochloric acid. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web find the ph and the poh of the solution. A 0 m solution of sodium hydroxide. Student will also familiarizing themselves with the. Ph and poh calculations worksheet #2. Names _____ date _____ class _____ find the [h 3 o+]. Web ph and poh calculations worksheet #2. Web up to 6% cash back we can then derive the poh from the ph value. A 0 m solution of sulfuric acid. A 0.0334 m solution of potassium. Web the ph range of acids and bases (along with common acids/bases). The ph is given by the equation. Student will also familiarizing themselves with the. Web find the ph and the poh of the solution. Web ph and poh calculations worksheet #2. A 0 m solution of sodium hydroxide. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Web the ph range of acids and bases (along with common acids/bases). Calculating the ph of acids.calculating the ph and poh of bases.this set includes four (5) pages of. Find the ph and the poh of the solution. The ph is given by the equation. The ph is given by the equation. Web find the ph and the poh of the solution. Web up to 6% cash back we can then derive the poh from the ph value. A 0 m solution of sodium hydroxide. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Show your work and use correct sig. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. The ph is given by the equation. Names _____ date _____ class _____ find the [h 3 o+]. Names __________________________________________ date ____________ class _______. A 0.023 m solution of hydrochloric acid. Find the ph and the poh of the solution. Ph and poh calculations worksheet #2. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. The ph and poh of a neutral solution. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. First, we'll walk through the possible approaches for calculating [h₃o⁺] from poh. The ph is given by the equation. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of. Web 14.00 = ph + poh. Web ph and poh calculations. Web find the ph and the poh of the solution. At this temperature, then, neutral solutions exhibit ph = poh =. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. Calculating the ph of acids.calculating the ph and poh of bases.this set includes four (5) pages of. Web 14.00 = ph + poh. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Web up to 6% cash back we can then derive the poh from the ph value. A 0.0334 m solution of potassium. Web free collection of ph and poh calculation worksheets for students if you want to calculate the ph , you have to take the negative log of the hydronium ion concentration. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. Ph and poh calculations worksheet #2. Web find the ph and the poh of the solution. A 0.023 m solution of hydrochloric acid. The ph is given by the equation. A 0.023 m solution of hydrochloric acid. Names __________________________________________ date ____________ class _______. A 0 m solution of sulfuric acid. Student will also familiarizing themselves with the. Concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. The ph and poh of a neutral solution. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Fill in the missing information in the table below. A 0 m solution of sodium hydroxide. The ph is given by the equation. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Concentrations of hydrogen to ph, concentrations of oh to ph all types of ph and poh calculations. Ph and poh calculations worksheet #2. As was shown in example 14.1, the hydronium ion molarity in pure water (or any neutral solution) is 1.0 × 10 −7 m at 25 °c. A 0.023 m solution of hydrochloric acid. At this temperature, then, neutral solutions exhibit ph = poh =. Web find the ph and the poh of the solution. Calculate the ph and the poh of each of the following solutions at 25 °c for. Web up to 6% cash back we can then derive the poh from the ph value. Web in this video, we'll solve for [h₃o⁺] and ph in two different worked examples. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web ph and poh calculations. Student will also familiarizing themselves with the. Show your work and use correct sig.(pH & pOH) of a Solution Chemistry, Biochemistry, Mcat
20++ Ph And Poh Worksheet Answer Key Worksheets Decoomo
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
calculating ph and poh worksheet
Ph And Poh Worksheet Answers Pdf → Waltery Learning Solution for Student
Ph and Poh Worksheet Free Worksheet
Ph And Poh Worksheet
Ph And Poh Worksheet Answers Kayra Excel
Ph And Poh Worksheet Answers
A 0.0334 M Solution Of Potassium.
Names __________________________________________ Date ____________ Class _______.
The Ph And Poh Of A Neutral Solution.
Web Ph = − Log[H 3O +] = − Log(4.9 × 10 − 7) = 6.31.
Related Post: