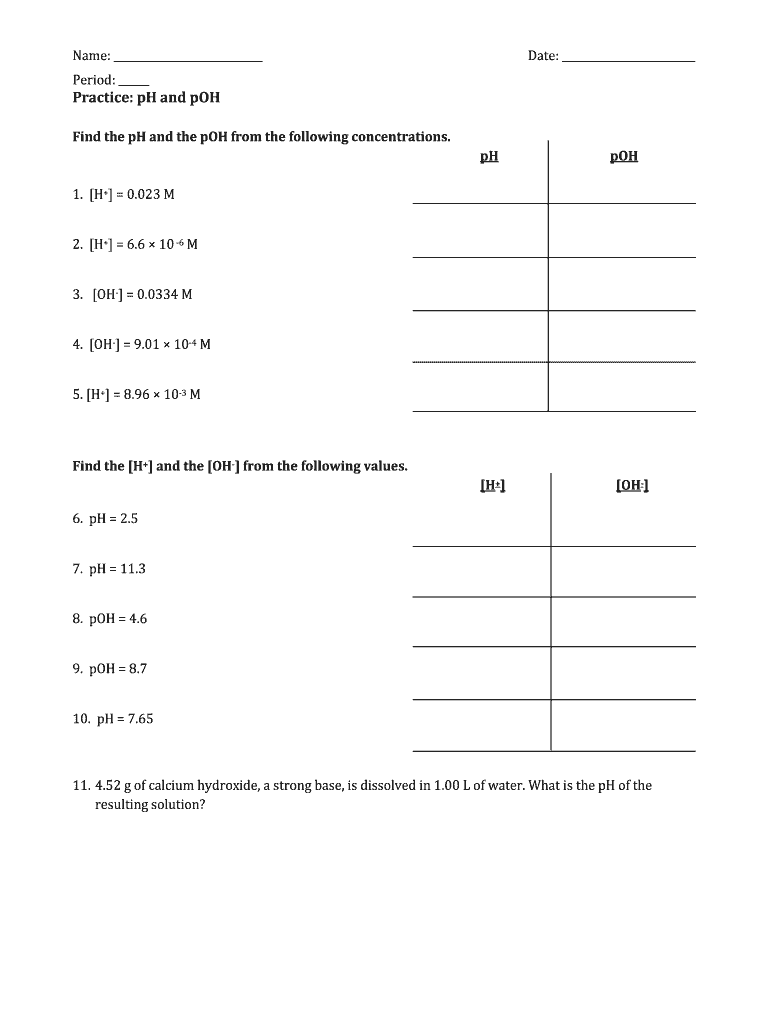
Ph And Poh Calculations Worksheet
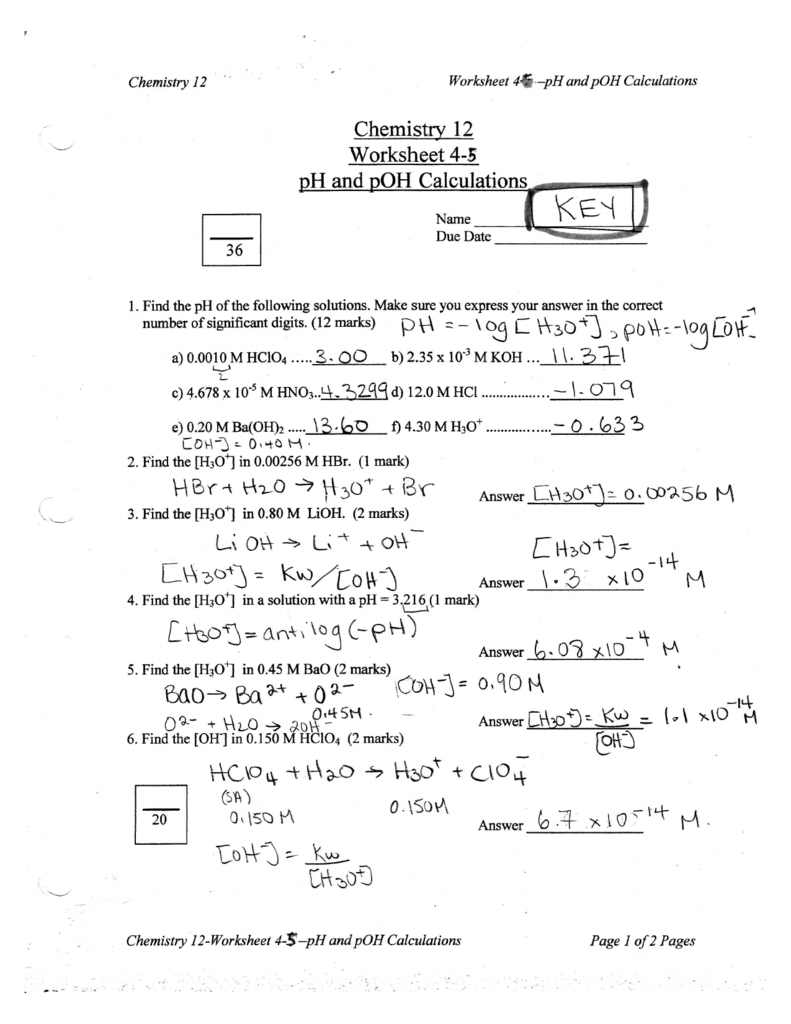
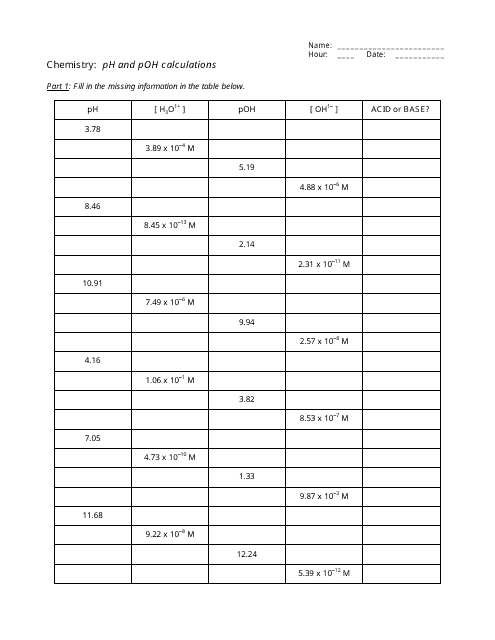
Ph And Poh Calculations Worksheet - Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. A 0.023 m solution of hydrochloric acid. Web up to 6% cash back we can then derive the poh from the ph value. Web find the ph and the poh of the solution. Fill in the missing information in the table below. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. If you are struggling with ph and poh calculations , you have come to the right place. For example the ph of a solution is given by. Web calculate the poh by plugging the [oh −] into the equation. Web you find the ph by simply subtracting poh from 14. Student will also familiarizing themselves with the. A 0.0334 m solution of potassium. At this temperature, then, neutral solutions exhibit ph = poh =. For example the ph of a solution is given by. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? If you are struggling with ph and poh calculations , you have come to the right place. Calculate the ph by rearranging and plugging in the poh: Poh = −log [ oh −] = −log ( 4.9. Web up to 6% cash back we can then derive the poh from the ph value. Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. Determine the ph of each solution of the following bases. A 0.023 m solution of hydrochloric acid. Web ph. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web you find the ph by simply subtracting poh from 14. Calculate the ph by rearranging and plugging in the poh: If you are struggling with ph and poh calculations , you have come to the right place. Student will also familiarizing themselves with the. Web up to 6% cash back we can then derive the poh from the ph value. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. A 0.023 m solution of hydrochloric acid. A 0.0334 m solution of potassium. Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have. At this temperature, then, neutral solutions exhibit ph = poh =. Web up to 6% cash back we can then derive the poh from the ph value. Calculate the ph by rearranging and plugging in the poh: At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Determine the ph of each solution of the following bases. A 0.0334 m solution of potassium. We have collected a myriad of high. A 0.023 m solution of hydrochloric acid. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Calculate the ph by rearranging and plugging in the poh: For example the ph of a solution is given by. Web calculate the poh by plugging the [oh −] into the equation. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. The ph is given by the equation. Calculate the ph by rearranging and plugging in the poh: Student will also familiarizing themselves with the. At this temperature, then, neutral solutions exhibit ph = poh =. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. Since hydrochloric acid is. At this temperature, then, neutral solutions exhibit ph = poh =. Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. For example the ph of a solution is given by. Web ph = −log [ h 3 o +] = −log ( 4.9 ×. Calculate the ph by rearranging and plugging in the poh: The ph is given by the equation. At this temperature, then, neutral solutions exhibit ph = poh =. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Web calculate the poh by plugging the [oh −] into the equation. A 0.023 m solution of hydrochloric acid. Fill in the missing information in the table below. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web up to 6% cash back we can then derive the poh from the ph value. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Determine the ph of each solution of the following bases. Web here is a very useful worksheet on calculating the ph and poh of acids and bases.i have included a log table for you convenience. If you are struggling with ph and poh calculations , you have come to the right place. Web find the ph and the poh of the solution. A 0.0334 m solution of potassium. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. We have collected a myriad of high. For example the ph of a solution is given by. Web find the ph and the poh of the solution. Student will also familiarizing themselves with the. For example the ph of a solution is given by. If you are struggling with ph and poh calculations , you have come to the right place. At this temperature, then, neutral solutions exhibit ph = poh =. Determine the ph of each solution of the following bases. Web if the ph is 11.64 and you have 2.55 l of solution, how many grams of calcium hydroxide are in the solution? Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. Web you find the ph by simply subtracting poh from 14. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Fill in the missing information in the table below. Web up to 6% cash back we can then derive the poh from the ph value. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic.Ph and poh calculations worksheet Fill out & sign online DocHub
30 Ph and Poh Worksheet Education Template
Chem 12 WS4.5 (pH and pOH)
Ph and Poh Calculations Chemistry Worksheet With Answers Download
Ph And Poh Calculations Worksheet Ph and Poh Worksheet
Ph And Poh Calculations Worksheet Thekidsworksheet
30 Ph and Poh Worksheet Education Template
pH and pOH Calculations Worksheet Google Drive
Chemistry Ph And Poh Calculations Worksheet Tomas Blog
ph and poh calculations worksheet
Calculate The Ph By Rearranging And Plugging In The Poh:
Web Calculate The Poh By Plugging The [Oh −] Into The Equation.
The Ph Is Given By The Equation.
A 0.023 M Solution Of Hydrochloric Acid.
Related Post:






.PNG)