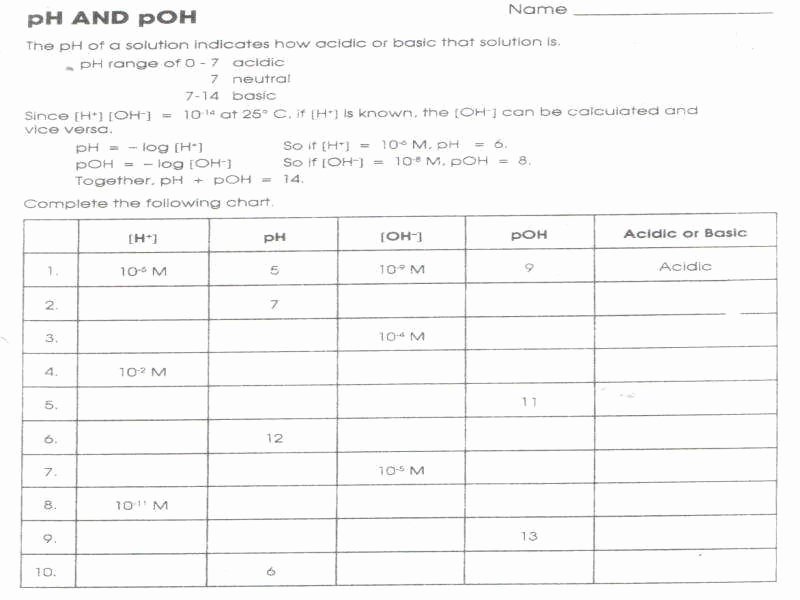
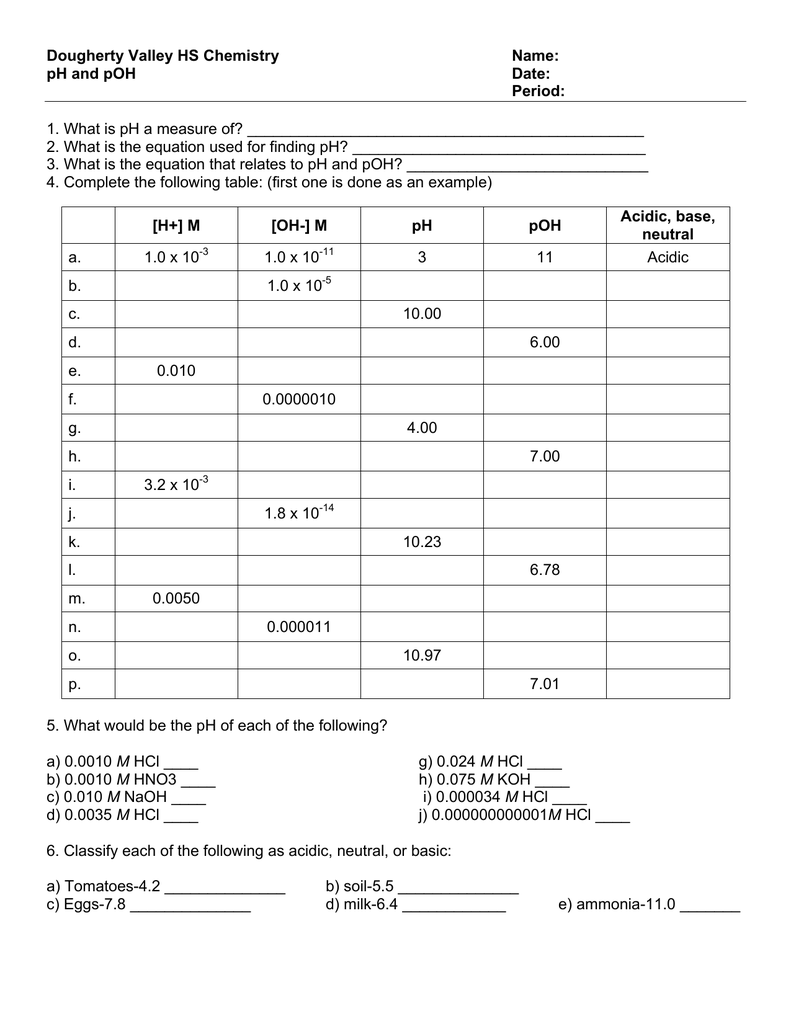
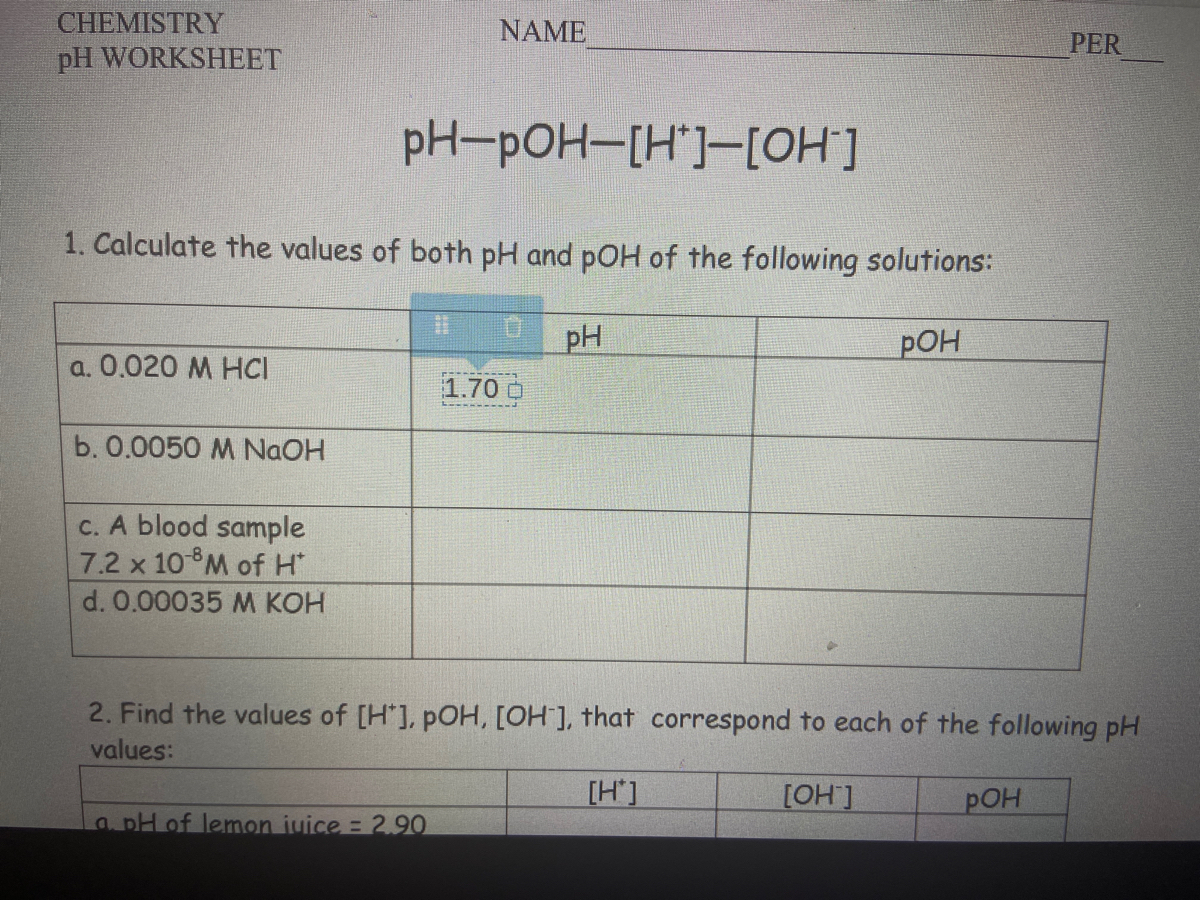
Ph And Poh Worksheet Answer Key
Ph And Poh Worksheet Answer Key - A 0.023 m solution of hydrochloric acid. Web naming acids worksheet (docx 15 kb) ph and poh (docx 15 kb) acid and base worksheet (doc 30 kb) acid base neutralization (doc 24 kb) ph indicators (doc 33. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Web introductory acid base worksheet (and complete solution answer key) starting with basic logarithms and ph (extending to poh, pka, pkb and pkw). Fill in the missing information in the table below. Ph = − log[h3o +]. Web find the ph and the poh of the solution. Web calculate the poh by plugging the [oh −] into the equation. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Uopnps puy noá dn0jb jnoá ssnosya The ph is given by the equation. Web up to 6% cash back we can then derive the poh from the ph value. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. You express your answer in the correct number. Poh = − log[oh −] = − log(4.9 × 10 − 7) =. Web introductory acid base worksheet (and complete solution answer key) starting with basic logarithms and ph (extending to poh, pka, pkb and pkw). Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. Web calculate the poh by plugging the [oh −] into the equation. Web up to 6% cash back we can then derive. Fill in the missing information in the table below. Web calculate the poh by plugging the [oh −] into the equation. Web find the ph and the poh of the solution. Poh = −log [ oh −] = −log ( 4.9 × 10 −7) = 6.31. Show your work and use correct sig. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web up to 6% cash back we can then derive the poh from the ph value. Web calculate the poh by plugging the [oh −] into the equation. A 0.023 m solution of hydrochloric acid. Web naming acids worksheet (docx 15 kb) ph and poh (docx 15 kb). Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web naming acids worksheet (docx 15 kb) ph and poh (docx 15 kb) acid and base worksheet (doc 30 kb) acid base neutralization (doc 24 kb) ph indicators (doc 33. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Fill in the missing information in the table below.. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Web introductory acid base worksheet (and complete solution answer key) starting with basic logarithms and ph (extending to poh, pka, pkb and pkw). Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Student will also familiarizing themselves with the. Since. Web naming acids worksheet (docx 15 kb) ph and poh (docx 15 kb) acid and base worksheet (doc 30 kb) acid base neutralization (doc 24 kb) ph indicators (doc 33. A 0.023 m solution of hydrochloric acid. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! At this temperature, then, neutral solutions exhibit ph = poh =.. A 0.023 m solution of hydrochloric acid. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web find the ph and the poh of the solution. Fill in the missing information in the table below. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Fill in the missing information in the table below. At this temperature, then, neutral solutions exhibit ph = poh =. A 0.023 m solution of hydrochloric acid. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Fill in the missing information in the table below. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. You express your answer in the correct number. Show your work and use correct sig. The ph is given by the equation. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Calculate the ph by rearranging and plugging in the poh: At this temperature, then, neutral solutions exhibit ph = poh =. Web ph, poh, k a & pk a worksheet calculate the ph of each of the following aqueous solutions and tell whether the solution is acidic, basic or neutral. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Web naming acids worksheet (docx 15 kb) ph and poh (docx 15 kb) acid and base worksheet (doc 30 kb) acid base neutralization (doc 24 kb) ph indicators (doc 33. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. Student will also familiarizing themselves with the. Fill in the missing information in the table below. Web calculate the poh by plugging the [oh −] into the equation. Web ph = − log[h 3o +] = − log(4.9 × 10 − 7) = 6.31. A 0.023 m solution of hydrochloric acid. Uopnps puy noá dn0jb jnoá ssnosya Show your work and use correct sig. Web up to 6% cash back we can then derive the poh from the ph value. Web introductory acid base worksheet (and complete solution answer key) starting with basic logarithms and ph (extending to poh, pka, pkb and pkw). Web find the ph and the poh of the solution. Fill in the missing information in the table below. Uot pp01,n u10jj saldurexa btllsn a.rnpaoo.ld noåj! Fill in the missing information in the table below. Poh = − log[oh −] = − log(4.9 × 10 − 7) = 6.31. Web calculate the poh by plugging the [oh −] into the equation. Web ph = −log [ h 3 o +] = −log ( 4.9 × 10 −7) = 6.31. Web 100tps.10] yo hd suopnps jo 01 pasgv.1 0 [jo anpa s! Web introductory acid base worksheet (and complete solution answer key) starting with basic logarithms and ph (extending to poh, pka, pkb and pkw). A 0.023 m solution of hydrochloric acid. At this temperature, then, neutral solutions exhibit ph = poh = 6.31, acidic. You express your answer in the correct number. Since hydrochloric acid is monoprotic, the concentration of the solution is equal to. Ph = − log[h3o +]. Student will also familiarizing themselves with the. Calculate the ph by rearranging and plugging in the poh: The ph is given by the equation. Fill in the missing information in the table below.PH and pOH worksheet
Ph and Poh Worksheet
Ph And Poh Worksheet Answers Worksheet List
Ph And Poh Worksheet Answer Key Kayra Excel
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
50 Ph and Poh Worksheet Answers Chessmuseum Template Library
Ph And Poh Worksheet Answers
34 Ph And Poh Worksheet Answers support worksheet
Ph And Poh Calculations Worksheet 2 Answer Key → Waltery Learning
pH and pOH Practice Worksheet
Poh = −Log [ Oh −] = −Log ( 4.9 × 10 −7) = 6.31.
Show Your Work And Use Correct Sig.
Uopnps Puy Noá Dn0Jb Jnoá Ssnosya
At This Temperature, Then, Neutral Solutions Exhibit Ph = Poh =.
Related Post:






/pHWorksheetAnswers-56a12dd93df78cf772682de3.png)