Rate Of Reaction Worksheet Answers
Rate Of Reaction Worksheet Answers - Suitable for additional science c3 or chemistry. Answer_____ b) calculate the rate of reaction in moles of zn consumed per second. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Calculate the mean rate of reaction from 0 to 30 seconds. When a solid produced by a chemical reaction separates from a solution it is called a. This is a chem worksheet that might have answers on it name: For the product being produced. If a reaction is to occur, reacting particles must first _____ and this _____ must be effective. C) the instantaneous rate of reaction at 30 seconds. Web pdf, 3.06 mb. Record the results in your data table and again calculate the average from the combined class data for each experiment. According to the collision theory, what 3 circumstances are needed for c 6 h 12 o 6. This is a chem worksheet that might have answers on it name: When a solid produced by a chemical reaction separates from a. A study of reaction _____ is called chemical _____. Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting rate of reactions (concentration of reactants, temperature, presence of a catalyst, surface area of reactants) and understanding graphs related to the reaction.suited for students. Finding and using the. Web a) calculate the rate of reaction in grams of zn consumed per second. Finding and using the laws which predict reaction rates. C) the instantaneous rate of reaction at 30 seconds. Record the results in your data table and again calculate the average from the combined class data for each experiment. In this worksheet, we will practice describing the. Web a) calculate the rate of reaction in grams of zn consumed per second. For the product being produced. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. When a solid produced by a chemical reaction separates from a solution it is called a. Suitable for. A sprinkling of hsw stuff as well and a grade ladder. Calculate the mean rate of reaction from 0 to 30 seconds. Web rate of reaction worksheet with answer. Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting rate of reactions (concentration of reactants, temperature, presence. For the product being produced. When slope = 0, rate = 0 = reaction is over. When a solid produced by a chemical reaction separates from a solution it is called a. Web always be equal to the total mass of the products. Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Web explore what makes a reaction happen by colliding atoms and molecules. For the product being produced. Web take a quick interactive quiz on the concepts in calculating rates of reaction: Finding and using the laws which predict reaction rates. Calculate the mean rate of reaction from 0 to 30 seconds. Design experiments with different reactions, concentrations, and temperatures. Use the collision theory to deduce why rate changes when temperature, pressure, skip to document. When a solid produced by a chemical reaction separates from a solution it is called a. Reaction rate laws give an equation for finding the rate. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. What affects the rate of a reaction? For the product being produced. H 2 o + co 2 as the reaction proceeds ? Web a) calculate the rate of reaction in grams of zn consumed per second. It is measured in terms of the _____ of the reactants. In this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect the type of reagent and the surface area have on it. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Suitable for additional science c3 or. Web 2 worksheets consisting of 30 questions and answers related to calculating average rate of reactions, instantaneous rate of reactions, determining factors affecting rate of reactions (concentration of reactants, temperature, presence of a catalyst, surface area of reactants) and understanding graphs related to the reaction.suited for students. Calculate the mean rate of reaction from 0 to 30 seconds. In this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect the type of reagent and the surface area have on it. A) the average rate of reaction over the first 10 seconds. Web product recorded was 24.9 g after 30 seconds. Record the results in your data table and again calculate the average from the combined class data for each experiment. Design experiments with different reactions, concentrations, and temperatures. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. The reaction rate law is reaction rate = k [a] m [b] n where k is a constant. According to the collision theory, what 3 circumstances are needed for c 6 h 12 o 6. When surface area matters!—page 3 b. If a reaction is to occur, reacting particles must first _____ and this _____ must be effective. Web pdf, 3.06 mb. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. What happens to the concentrations of: Answer_____ c) write out the complete ionic equation for the reaction. For the product being produced. Some of the worksheets for this concept are rate of reaction work, work reaction rates name, name per work reaction rates, work integrated rate law, chemical kinetics reaction rates, kinetics practice supplemental work key determining, kinetics practice supplemental. Another article, rationalising rates, includes tips for teaching rates of reaction using graphs. A sprinkling of hsw stuff as well and a grade ladder. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. Design experiments with different reactions, concentrations, and temperatures. Answer_____ b) calculate the rate of reaction in moles of zn consumed per second. Use the collision theory to deduce why rate changes when temperature, pressure, skip to document. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Web a) calculate the rate of reaction in grams of zn consumed per second. Web rate of reaction worksheet with answer. What affects the rate of a reaction? C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. When surface area matters!—page 3 b. B) the average rate of reaction over the first 60 seconds. Web a series of free high school chemistry video lessons. Formula, graphs & examples or print the worksheet to practice offline. For the product being produced. Calculate the mean rate of reaction from 0 to 30 seconds. The process by which chemical bonds are broken and formed is called a.Worksheet Factors Effecting Rates of Reaction
13 Worksheet Reaction Rates Answer /
Reaction Rates Worksheet Answers Kayra Excel
Rates of Reaction Worksheet 7.2
Rates Of Reaction Worksheet Ivuyteq
Chemistry Rate of Reaction Worksheet Cloze Passage Teaching Resources
Answers Rates of Reaction
13 Best Images of Worksheet Reaction Rates Answer Worksheet Measuring
Rate of Reaction changing the rate labs Gcse science revision, Gcse
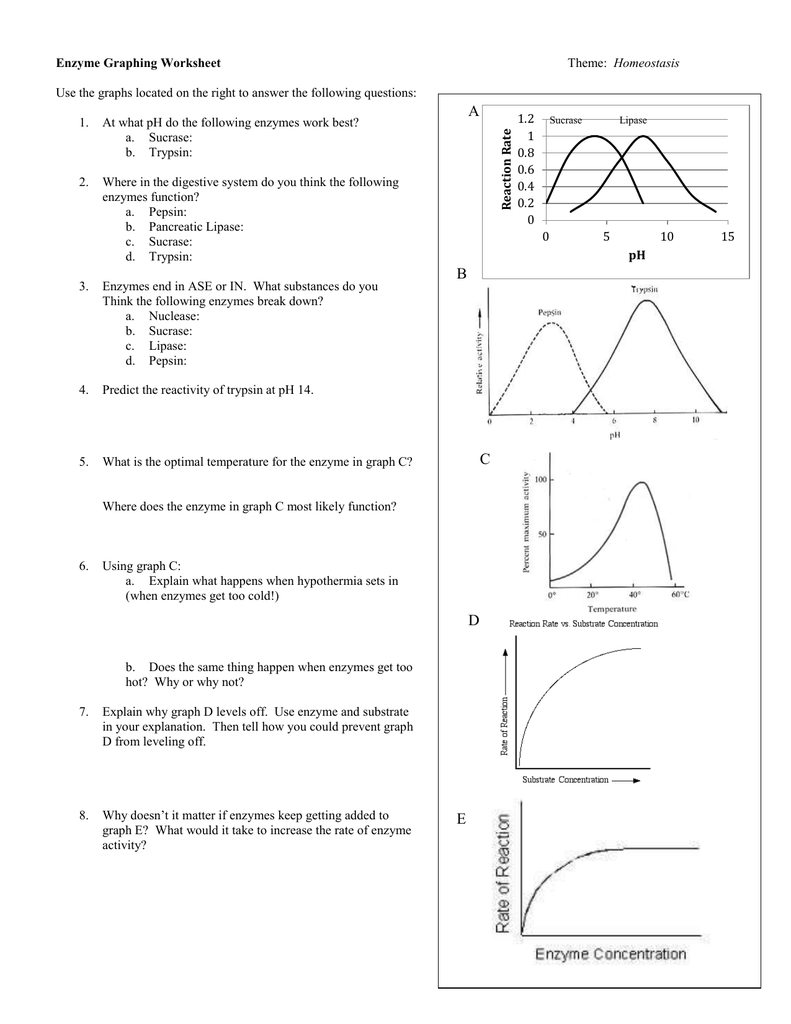
Enzyme Reactions Worksheet Answers
What Happens To The Concentrations Of:
A Sprinkling Of Hsw Stuff As Well And A Grade Ladder.
Web 2 Worksheets Consisting Of 30 Questions And Answers Related To Calculating Average Rate Of Reactions, Instantaneous Rate Of Reactions, Determining Factors Affecting Rate Of Reactions (Concentration Of Reactants, Temperature, Presence Of A Catalyst, Surface Area Of Reactants) And Understanding Graphs Related To The Reaction.suited For Students.
Convert The Reaction Times Into Average Reaction Rates Using The Equation Given Below.
Related Post: