Reaction Rate Worksheet
Reaction Rate Worksheet - Web a student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. It is measured in terms of the. In this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect the type of reagent and the surface. Enzymes are in molds and bacteria that spoil food. Web rate of reaction worksheets. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products,. Web in this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect of heat on the rate of reaction using the collision theory. C 6 h 12 o. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. Web the rate of reaction is simply a measure of how fast a reaction proceeds, which we can determine through monitoring how fast a reactant is used up or how fast a product. It is measured in. The student had recorded an initial reactant mass of 27 g. It is measured in terms of the. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample data to determine rate law, evaluate reaction mechanism to determine. A study of reaction _____ is called chemical _____. Web worksheets are name per work. Factor affected rate of reaction. It is measured in terms of the. It is measured in terms of the. A reaction has the experimental rate law, rate = k[a]2. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. It is measured in terms of the. C 6 h 12 o. Design experiments with different reactions, concentrations, and temperatures. It is measured in terms of the. Web explore what makes a reaction happen by colliding atoms and molecules. C 6 h 12 o. Reaction rate refers to how quickly or slowly the reactants disappear and the products appear. Explain, using your knowledge of factors affecting the. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample. A reaction has the experimental rate law, rate = k[a]2. Reaction rate refers to how quickly or slowly the reactants disappear and the products appear. The student had recorded an initial reactant mass of 27 g. C 6 h 12 o. Web rate of reaction worksheets. Web worksheets are name per work reaction rates, chemistry 12 work 1 1, work reaction rates name, types of reactions work, chapter 16 reaction rates, science term 1 rates of. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample data to determine rate law, evaluate reaction mechanism to determine. A reaction has. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. The student had recorded an initial reactant mass of 27 g. Web in this practice worksheet students will determine the affect of. It is measured in terms of the. Web a student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Web rate of reaction worksheets. Web the rate of reaction is simply a measure. Explain, using your knowledge of factors affecting the. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample data to determine rate law, evaluate reaction mechanism to determine. Factor affected rate of reaction. Web decreasing temperature (decreases, increases) the rate of reaction. A reaction has the experimental rate law, rate = k[a]2. A reaction has the experimental rate law, rate = k[a]2. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Web calculate the average reaction times from the combined class data that you will collect together with your teacher. Reaction rate laws give an equation for finding the rate of a reaction using the concentration of the reactants and the stoichiometric coefficients. Web explore what makes a reaction happen by colliding atoms and molecules. Web rate of reaction worksheets. Web the rate of reaction is simply a measure of how fast a reaction proceeds, which we can determine through monitoring how fast a reactant is used up or how fast a product. A study of reaction _____ is called chemical _____. What happens to the concentrations of: Explain, using your knowledge of factors affecting the. Web a study of reaction rate is called chemical kinetic. Factor affected rate of reaction. Enzymes are in molds and bacteria that spoil food. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample data to determine rate law, evaluate reaction mechanism to determine. It is measured in terms of the. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. C 6 h 12 o. In this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect the type of reagent and the surface. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products,. It is measured in terms of the. Web experimentally determined rate law expressions show how the rate of a reaction depends upon the concentrations of the reactants and sometimes the products,. C 6 h 12 o. The student had recorded an initial reactant mass of 27 g. Reaction rate refers to how quickly or slowly the _____ disappear and the _____ appear. A study of reaction _____ is called chemical _____. Reaction rate refers to how quickly or slowly the reactants disappear and the products appear. C 6 h 12 o 6 (s) + 6 o 2 (g) 6 h 2 o (g) + 6 co 2 (g) 1. Web in this worksheet, we will practice describing the rate of a chemical reaction and explaining the effect of heat on the rate of reaction using the collision theory. Web a study of reaction rate is called chemical kinetic. It is measured in terms of the. Web a student investigated the rate of reaction between calcium carbonate (marble chips) and hydrochloric acid. It is measured in terms of the. Design experiments with different reactions, concentrations, and temperatures. Web in this practice worksheet students will determine the affect of changes on reaction rate, use sample data to determine rate law, evaluate reaction mechanism to determine. Web calculate the average reaction times from the combined class data that you will collect together with your teacher. Explain, using your knowledge of factors affecting the.Worksheet Grade 8 Reaction Rate Chemical Reactions
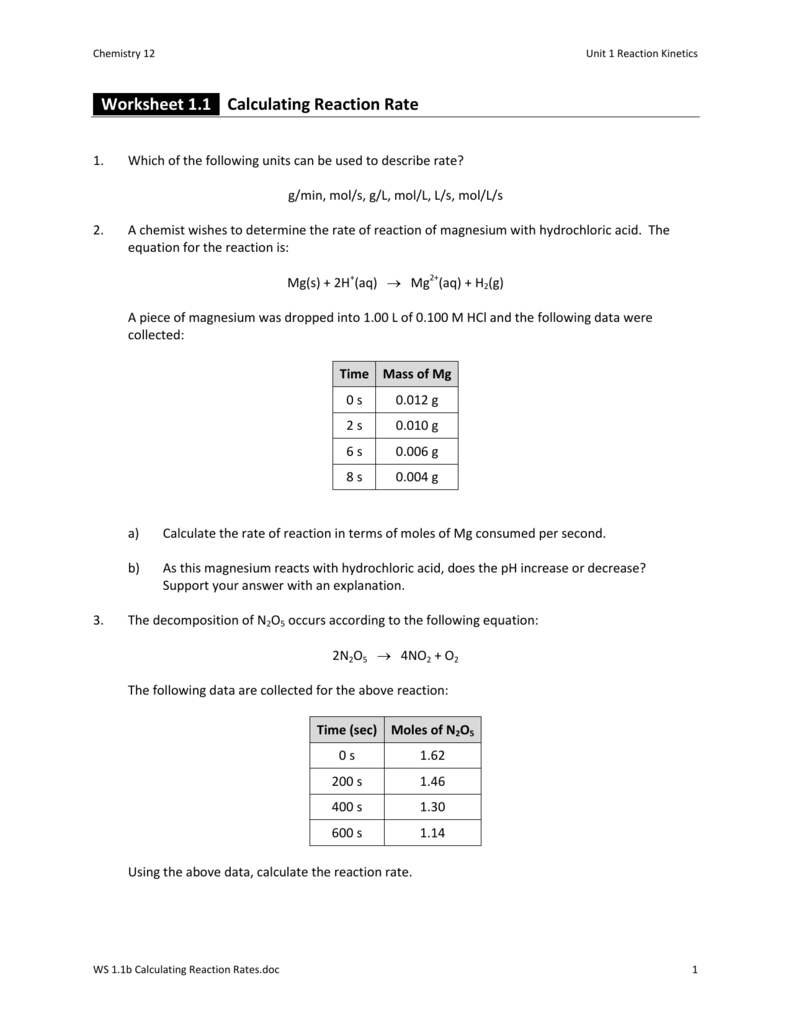
Worksheet 1.1 Calculating Reaction Rate
13 Worksheet Reaction Rates Answer /
Chemistry Reaction Rates Worksheet Wildseasonthegame —
Rates Of Reaction Worksheet Promotiontablecovers
13 Best Images of Worksheet Reaction Rates Answer Worksheet Measuring
️Chemical Reaction Rates Worksheet Free Download Gambr.co
Rates Of Reaction Worksheet Sky Hut
Rate of Reaction Practical Home Learning Worksheet GCSE Teaching
️Worksheet Reaction Rates Answer Key Free Download Goodimg.co
In This Worksheet, We Will Practice Describing The Rate Of A Chemical Reaction And Explaining The Effect The Type Of Reagent And The Surface.
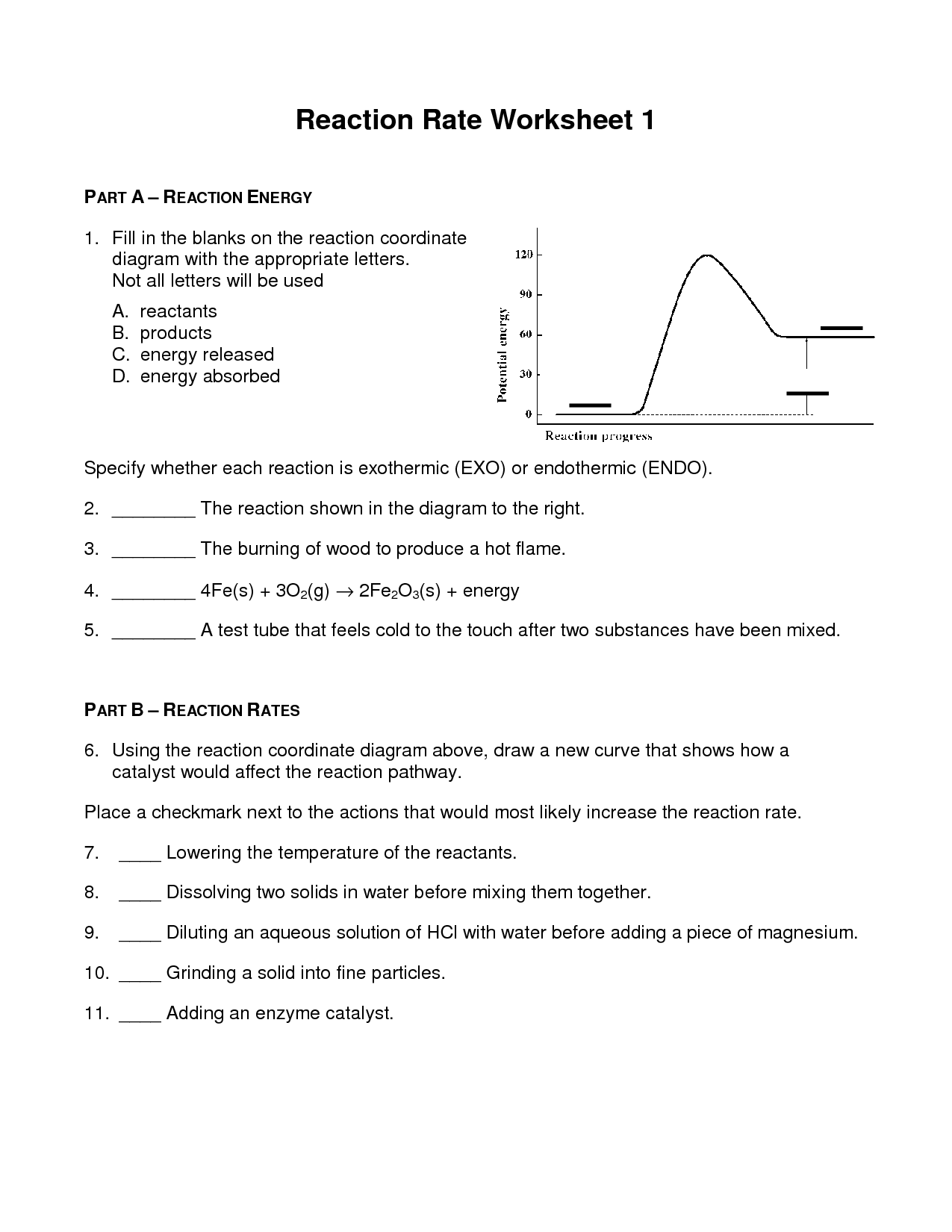
Web Decreasing Temperature (Decreases, Increases) The Rate Of Reaction.
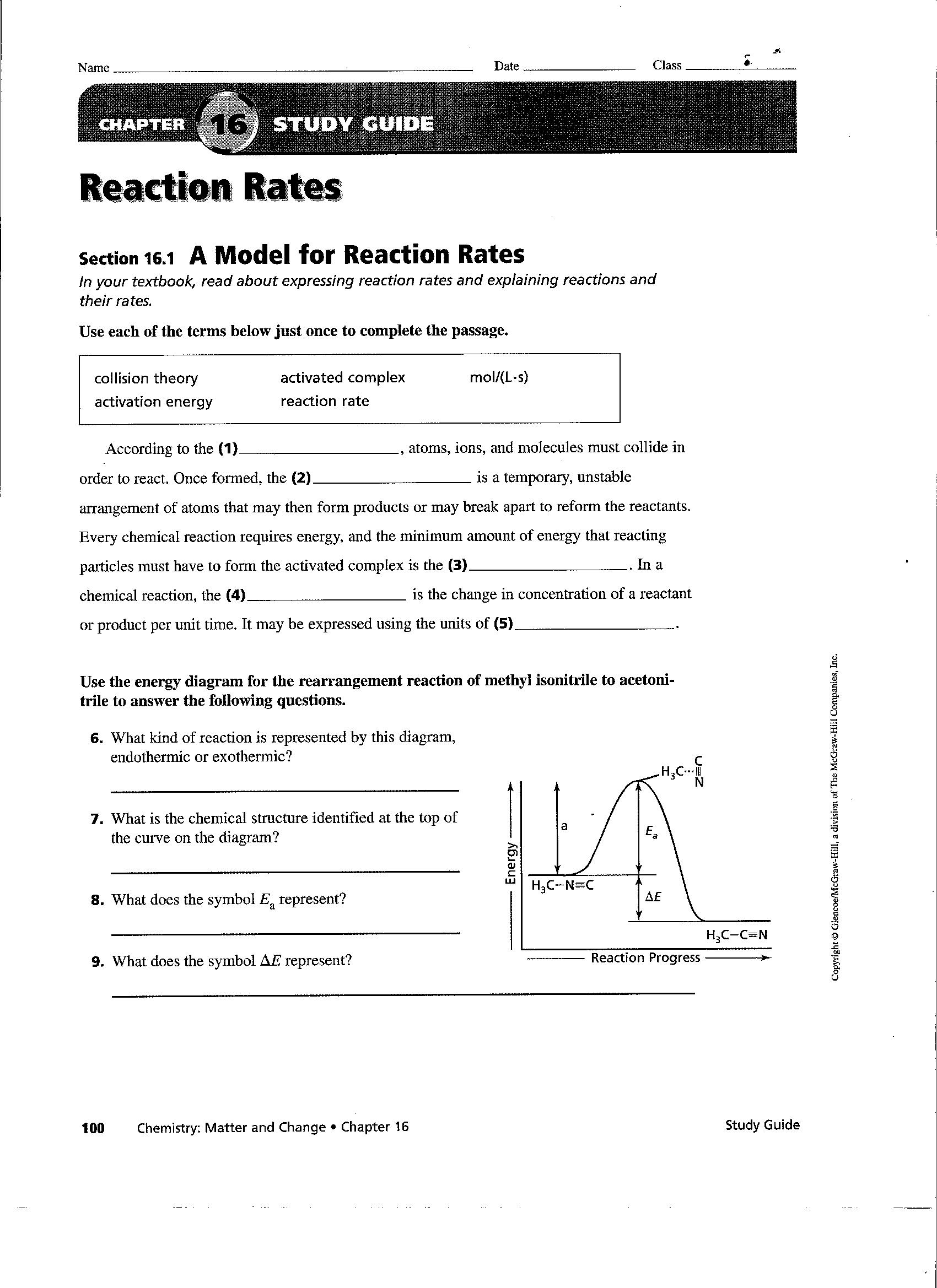
Web Explore What Makes A Reaction Happen By Colliding Atoms And Molecules.
Reaction Rate Laws Give An Equation For Finding The Rate Of A Reaction Using The Concentration Of The Reactants And The Stoichiometric Coefficients.
Related Post: