Solubility Guidelines For Aqueous Solutions Worksheet Answers
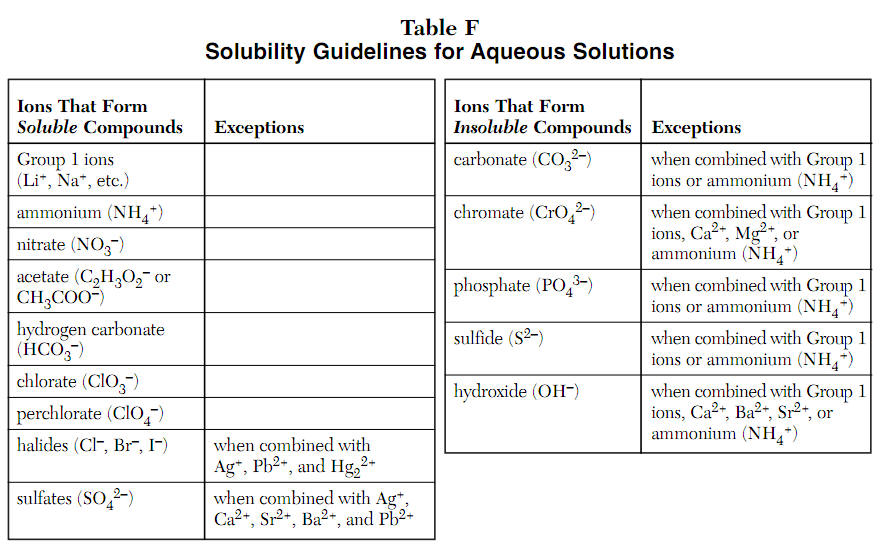
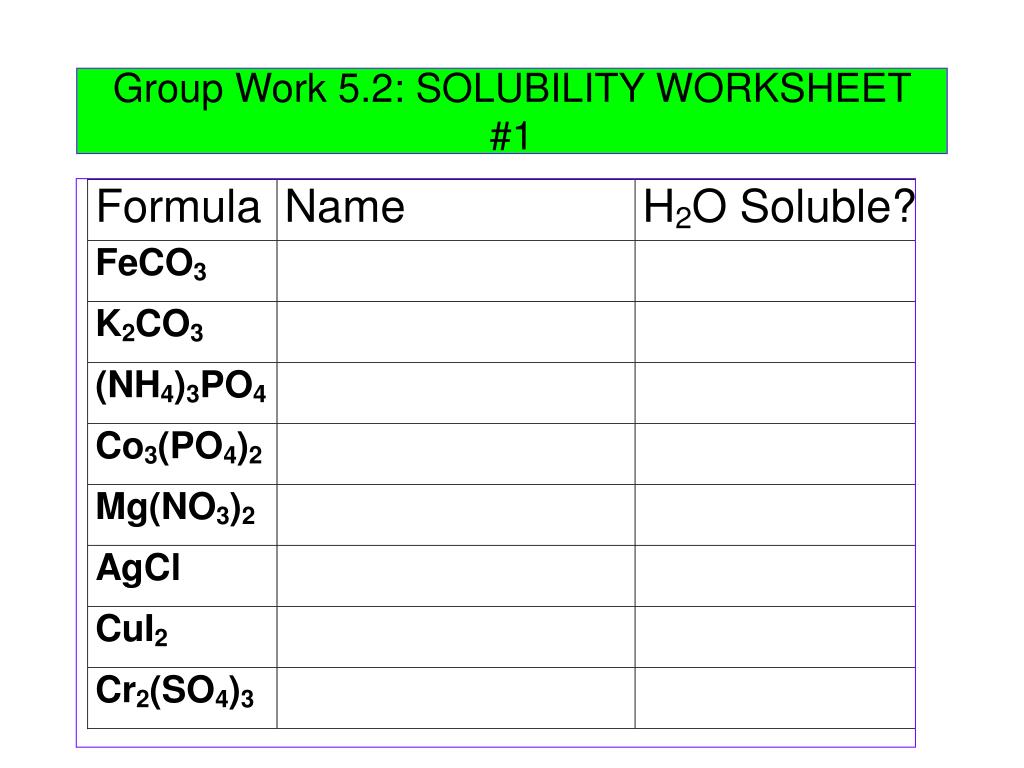
Solubility Guidelines For Aqueous Solutions Worksheet Answers - Therefore, we will consider the solution process more closely for aqueous solutions. Web encountered solutions are aqueous, i.e., in water. When the salt is added. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. Use the general water solubility guidelines to determine if the following solutes will dissolve in water. Aqueous solution solute and solvent, concentration units, saturated unsaturated supersaturated and dilution of solution, solubility, solutions suspension. If given the solubility product constant for a salt, we can determine the solubility for the salt as well. Web set 1 — solubility guidelines for aqueous solutions according to table f, which of these salts is least soluble in water? Web chemistry table f solubility guidelines for aqueous solutions. Web • it is very important that you know these guidelines and how to apply them in reactions. In order to do this, we need to use an ice table and the equilibrium constant expression. Web solubility rules reference table f solubility guidelines for aqueous solution. 434 views apr 7, 2021 chemistry videos. Web worksheet 6 — displacement reactions and acid/base reactions displacement reactions are those in which ions recombine in solution. Web set 1 — solubility guidelines. When the salt is added. 434 views apr 7, 2021 chemistry videos. Web • it is very important that you know these guidelines and how to apply them in reactions. I put the solvent in the solute and it dissolved. Web set 1 — solubility guidelines for aqueous solutions according to table f, which of these salts is least soluble. Web the introduction to table f worksheet consists of four pages:page 1: 2) all common compounds of the group ia metal ions and the ammonium ion are water. Web chemistry questions and answers; I dissolved the solvent and it would not mix. Therefore, we will consider the solution process more closely for aqueous solutions. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. Aqueous solution solute and solvent, concentration units, saturated unsaturated supersaturated and dilution of solution, solubility, solutions suspension. Web • it is very important that you know these guidelines and how to apply them in reactions. I dissolved the solvent and it would not mix. Therefore, we will consider. In order to do this, we need to use an ice table and the equilibrium constant expression. Web • it is very important that you know these guidelines and how to apply them in reactions. Web chemistry questions and answers; Use the solubility rules to predict whether or not the sollowing salts should dinssolve in water a. Web the general. Web insoluble, only soluble when combined with group 1 ions, ammonium, or ca²⁺, mg²⁺ If the products of this recombination are insoluble in water, a solid precipitate wili 434 views apr 7, 2021 chemistry videos. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. Terms in this set (14) group 1 ions (li+, na+, etc.) soluble: (3) fec12 (l) licl (2) rbcl opbc12 which compound is insoluble in water? In the beakers shown, use symbols to represent the appropriate compounds. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. In order to do this, we need to use an ice table and the equilibrium constant expression. Be able to predict the solubility or. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. Use the general water solubility guidelines to determine if the following solutes will dissolve in water. Terms in this set (14) group 1 ions (li+, na+, etc.) soluble: Web • it is very important that you know these guidelines and how to apply them in reactions. (3) fec12. Aqueous solution solute and solvent, concentration units, saturated unsaturated supersaturated and dilution of solution, solubility, solutions suspension. Web encountered solutions are aqueous, i.e., in water. Web solubility rules reference table f solubility guidelines for aqueous solution. Web types of solutions, freezing point depression with applications, dissolving at molecular level, effect of temperature and pressure on dissolving, solubility curve analysis, selective. Web chemistry table f solubility guidelines for aqueous solutions. Web solubility rules reference table f solubility guidelines for aqueous solution. Aqueous solution solute and solvent, concentration units, saturated unsaturated supersaturated and dilution of solution, solubility, solutions suspension. Web the general water solubility guidelines to determine if the following solutes will dissolve in water.aqueous solutions worksheetpeptides containing 50% or more hydrophobic. Be able to predict the solubility or insolubility of simple ionic compounds in water; Web set 1 — solubility guidelines for aqueous solutions according to table f, which of these salts is least soluble in water? Web chemistry questions and answers; Web solubility rules reference table f solubility guidelines for aqueous solution. Web the general water solubility guidelines to determine if the following solutes will dissolve in water.aqueous solutions worksheetpeptides containing 50% or more hydrophobic residues (w, l, i, f, m, v, y, p, a) are generally poorly soluble in aqueous solutions. I put the solvent in the solute and it dissolved. Which of the following sentences concerning solubility is phrased correctly? Web encountered solutions are aqueous, i.e., in water. Web what is the concentration of an aqueous sodium bromide solution with 0.45 moles of solute dissolved in 920 ml of water? Web worksheet 6 — displacement reactions and acid/base reactions displacement reactions are those in which ions recombine in solution. Web insoluble, only soluble when combined with group 1 ions, ammonium, or ca²⁺, mg²⁺ Use the general water solubility guidelines to determine if the following solutes will dissolve in water. In the beakers shown, use symbols to represent the appropriate compounds. 434 views apr 7, 2021 chemistry videos. Web chemistry table f solubility guidelines for aqueous solutions. When the salt is added. 2) all common compounds of the group ia metal ions and the ammonium ion are water. (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. (3) fec12 (l) licl (2) rbcl opbc12 which compound is insoluble in water? If the products of this recombination are insoluble in water, a solid precipitate wili 434 views apr 7, 2021 chemistry videos. Use the solubility rules to predict whether or not the sollowing salts should dinssolve in water a. (3) fec12 (l) licl (2) rbcl opbc12 which compound is insoluble in water? Terms in this set (14) group 1 ions (li+, na+, etc.) soluble: In the beakers shown, use symbols to represent the appropriate compounds. Be able to predict reactions of mixed. Be able to predict the solubility or insolubility of simple ionic compounds in water; (3) kc103 1 bas04 (2) cacr04 (4) which ion, when combined with. Web encountered solutions are aqueous, i.e., in water. Web set 1 — solubility guidelines for aqueous solutions according to table f, which of these salts is least soluble in water? In order to do this, we need to use an ice table and the equilibrium constant expression. 2) all common compounds of the group ia metal ions and the ammonium ion are water. I put the solvent in the solute and it dissolved. Which of the following sentences concerning solubility is phrased correctly? Web solubility rules reference table f solubility guidelines for aqueous solution. Topic 7_worksheet reactions in aqueous solution 1.Pivot Interactives Solubility Rules Answers Solved If The Solute Is
Pin on Homeschool Help
Solubility Curve Practice Worksheet Answers Solubility Curve Practice
Reactions In Aqueous Solutions Worksheet Answers worksheet
Solubility Rules Worksheet_Answers
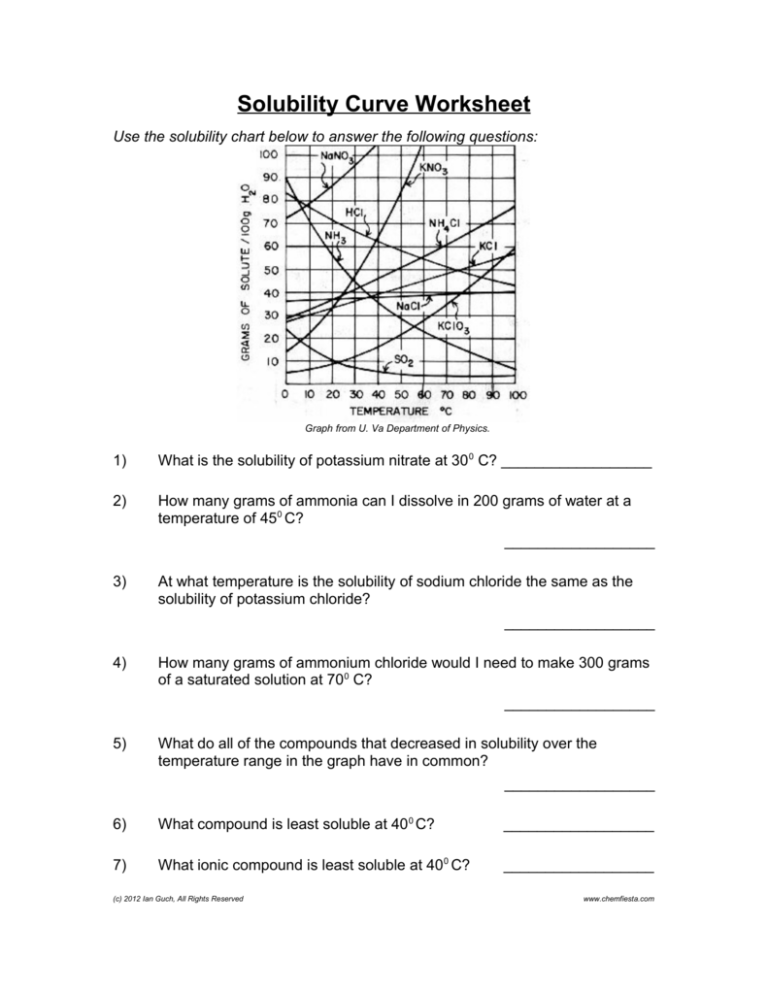
Solubility Curve Worksheet
Electrolytes
Reactions In Aqueous Solutions Worksheet Answers Lobo Black —
14 Best Images of Ion Worksheet High School Nomenclature Worksheet 2
PPT TOPIC V Ions in Aqueous Solutions PowerPoint Presentation, free
If Given The Solubility Product Constant For A Salt, We Can Determine The Solubility For The Salt As Well.
Use The General Water Solubility Guidelines To Determine If The Following Solutes Will Dissolve In Water.
Therefore, We Will Consider The Solution Process More Closely For Aqueous Solutions.
I Dissolved The Solvent And It Would Not Mix.
Related Post: