Solubility Polar Vs Nonpolar Worksheet Answers
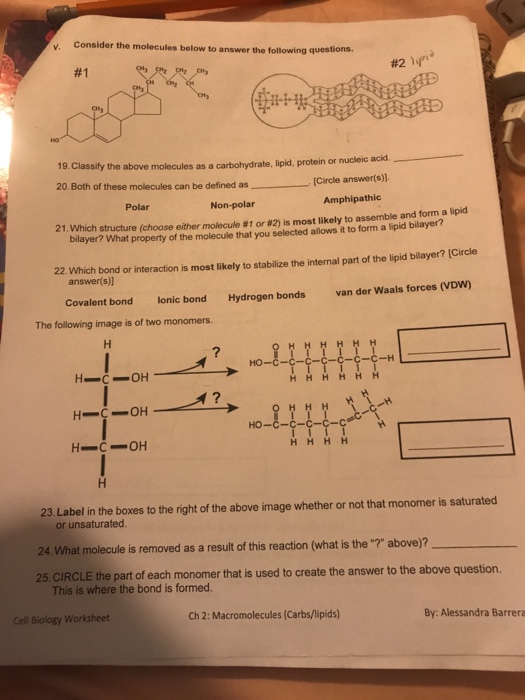
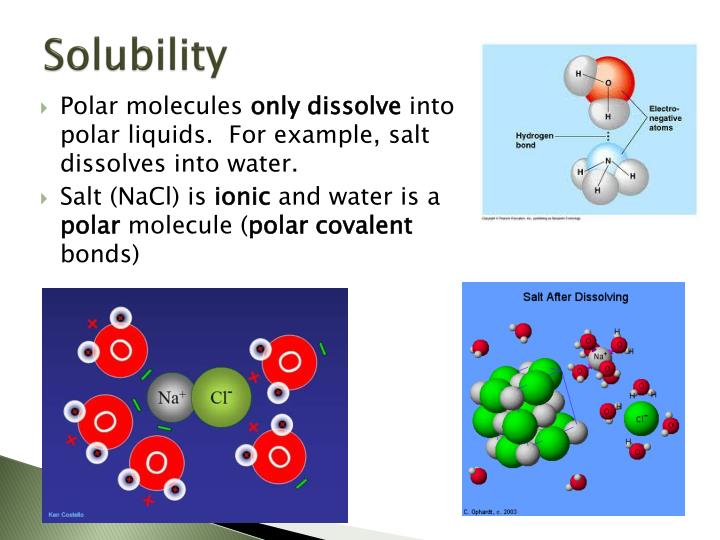
Solubility Polar Vs Nonpolar Worksheet Answers - Solubility can be defined as the tendency of one chemical substance to dissolve in a solvent and form a solution. This product comes in word format, so. Nonpolar molecules dissolve other nonpolar molecules. However, there is additional explaining to do when compared to the agcl example. The table below shows the structural formulas for three different compounds. Calculate the molar solubility (in mol/l) of a saturated solution of the substance. The polar solvent dissolves polar solutes and nonpolar solvent dissolves nonpolar solutes. Web the polarity of a molecule is important because it gives an idea of the solubility of the molecules in solvents. The solute can be a solid, a liquid, or even a gas and the solvent can be made up of almost any type of molecule. Web up to 24% cash back solubility (polar vs. Web hw #2 solubility (polar vs. That is, polar substances dissolve _____ (polar or nonpolar?) substances, and nonpolar substances dissolve _____ substances. Chloroform is “nonpolar” because it has a low dielectric constant. Generally, “like dissolves like.” polar molecules dissolve other polar molecules and ionic compounds. Nonpolar) name 'penerally, m like dissolves like. polar molecules dissolve other polar molecules and ionic. However, there is additional explaining to do when compared to the agcl example. The solute can be a solid, a liquid, or even a gas and the solvent can be made up of almost any type of molecule. Web up to 24% cash back created date: Solubility can be defined as the tendency of one chemical substance to dissolve in. This is an ionic salt and hence, it will be soluble in water only. I put the solvent in the solute and it dissolved. Alcohols, which have characteristics of both, tend to dissolve in both types of solvents, but will not dissolve ionic solids, check the appropriate. Web up to 24% cash back solubility (polar vs. Alcohol being polar, does. Some substances will be soluble in polar. Alcohol being polar, does not dissolves ionic salt in it. Covalent solubility uses, as it usually dissolves. This product comes in word format, so. Web hw #2 solubility (polar vs. Solubility depends on polarity and nonpolarity. Calculate the molar solubility (in mol/l) of a saturated solution of the substance. Web up to 24% cash back created date: I dissolved the solvent and it would not mix. In addition, connections with different polarities will be intractable in each other. This is an ionic salt and hence, it will be soluble in water only. This worksheet will test their knowledge. This means that substances with the same type of polarity will be soluble in each other. Can they predict its solubility/miscibility with other polar and nonpolar compounds? Which of the following sentences concerning solubility is phrased correctly? It also is a great intro into the forces of cohesion and adhesion. Web up to 24% cash back solubility (polar vs. It allows students to have real firsthand phenomena to experience and pose questions about. Which of the following sentences concerning solubility is phrased correctly? Web the polarity of a molecule is important because it gives an idea of. Nonpolar) name 'penerally, m like dissolves like. polar molecules dissolve other polar molecules and ionic compounds, nonpotar molecules dissolve other nonpolar molecules. Calculate the molar solubility (in mol/l) of a saturated solution of the substance. I put the solvent in the solute and it dissolved. Alcohols, which have characteristics of both, tend to dissolve in both types of solvents, but. It also is a great intro into the forces of cohesion and adhesion. It allows students to have real firsthand phenomena to experience and pose questions about. Web to determine if a molecule is polar or nonpolar, it is frequently useful to look at lewis structures. Alcohol being polar, does not dissolves ionic salt in it. Alcohols, which have characteristics. Web hw #2 solubility (polar vs. Nonpolar) check thea ro riate columns as to whether the solute is soluble in a olar or nonpolar solvent. It also is a great intro into the forces of cohesion and adhesion. Which of the following sentences concerning solubility is phrased correctly? Covalent solubility uses, as it usually dissolves. The table below shows the structural formulas for three different compounds. Web up to 24% cash back created date: I put the solvent in the solute and it dissolved. That is, polar substances dissolve _____ (polar or nonpolar?) substances, and nonpolar substances dissolve _____ substances. This product comes in word format, so. It also is a great intro into the forces of cohesion and adhesion. I dissolved the solvent and it would not mix. Calculate the molar solubility (in mol/l) of a saturated solution of the substance. Web up to 24% cash back solubility (polar vs. Solubility can be defined as the tendency of one chemical substance to dissolve in a solvent and form a solution. This means that substances with the same type of polarity will be soluble in each other. Chloroform is “nonpolar” because it has a low dielectric constant. It allows students to have real firsthand phenomena to experience and pose questions about. Covalent solubility uses, as it usually dissolves. The solute can be a solid, a liquid, or even a gas and the solvent can be made up of almost any type of molecule. One key solubility rule is that “like dissolves like”. Generally, “like dissolves like.” polar molecules dissolve other polar molecules and ionic compounds. Nonpolar) name 'penerally, m like dissolves like. polar molecules dissolve other polar molecules and ionic compounds, nonpotar molecules dissolve other nonpolar molecules. In addition, connections with different polarities will be intractable in each other. Worksheets are polar and non polar molecules, predicting polar non polar, lewis structures shapes and polarity, solubility miscibility, polar bonds supplemental work, chapter 7 practice work covalent bonds and molecular, lewis dot structures and vsepr, introduction to intermolecular forces. I put the solvent in the solute and it dissolved. Calculate the molar solubility (in mol/l) of a saturated solution of the substance. Which of the following sentences concerning solubility is phrased correctly? Web up to 24% cash back solubility (polar vs. Covalent solubility uses, as it usually dissolves. Nonpolar) check thea ro riate columns as to whether the solute is soluble in a olar or nonpolar solvent. Web the polarity of a molecule is important because it gives an idea of the solubility of the molecules in solvents. Solubility can be defined as the tendency of one chemical substance to dissolve in a solvent and form a solution. Nonpolar molecules dissolve other nonpolar molecules. Web hw #2 solubility (polar vs. Alcohol being polar, does not dissolves ionic salt in it. Alcohols, which have characteristics of both, tend to dissolve in both types of solvents, but will not dissolve ionic solids. Web up to 24% cash back solubility (polar vs. Some substances will be soluble in polar. Alcohols, which have characteristics of both, tend to dissolve in both types of solvents, but will not dissolve ionic solids, check the appropriate. In addition, connections with different polarities will be intractable in each other.️Bond Polarity Worksheet With Answers Free Download Gambr.co
Worksheet Polarity Of Bonds Answers
️Polar Or Nonpolar Molecules Worksheet Free Download Gambr.co
PPT Bonding PowerPoint Presentation ID3050946
Worksheet Polarity Of Bonds Answers
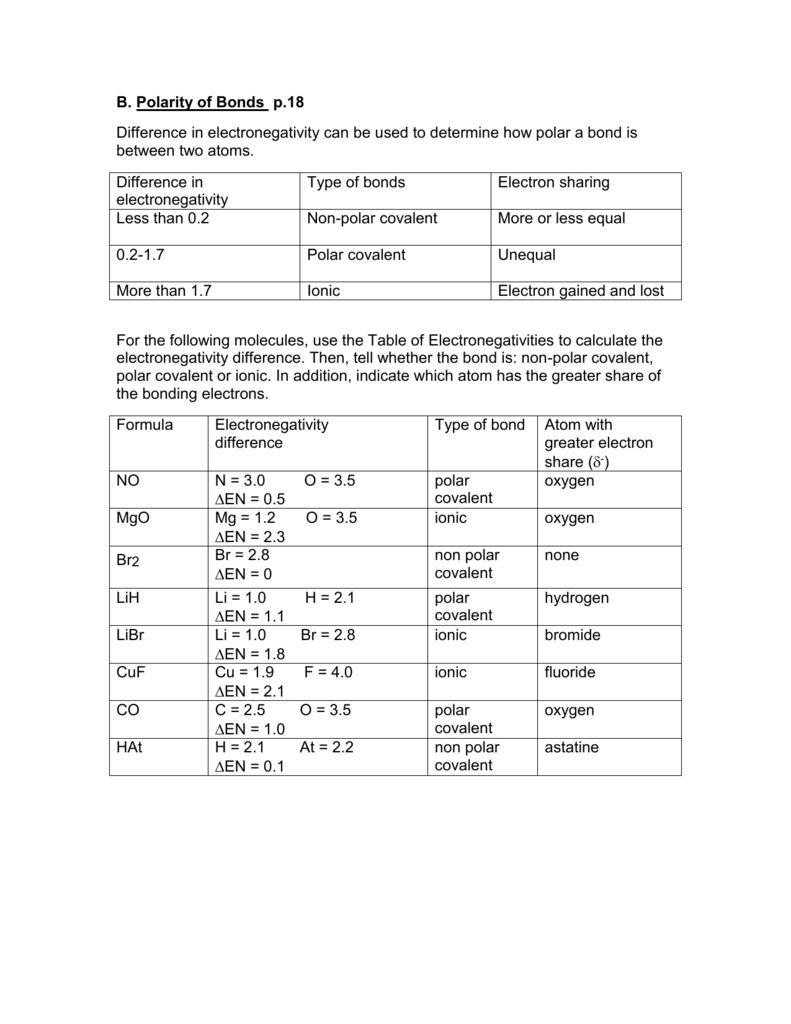
B Polarity Of Bonds P18 Difference In Electronegativity —
Vsepr And Polarity Worksheet Studying Worksheets
solubility (polar vs. Nonpolar)
Polar vs Nonpolar Lab
PPT Solubility and the Dissolving Process PowerPoint Presentation
Can They Predict Its Solubility/Miscibility With Other Polar And Nonpolar Compounds?
This Is An Ionic Salt And Hence, It Will Be Soluble In Water Only.
However, There Is Additional Explaining To Do When Compared To The Agcl Example.
The Solute Can Be A Solid, A Liquid, Or Even A Gas And The Solvent Can Be Made Up Of Almost Any Type Of Molecule.
Related Post: