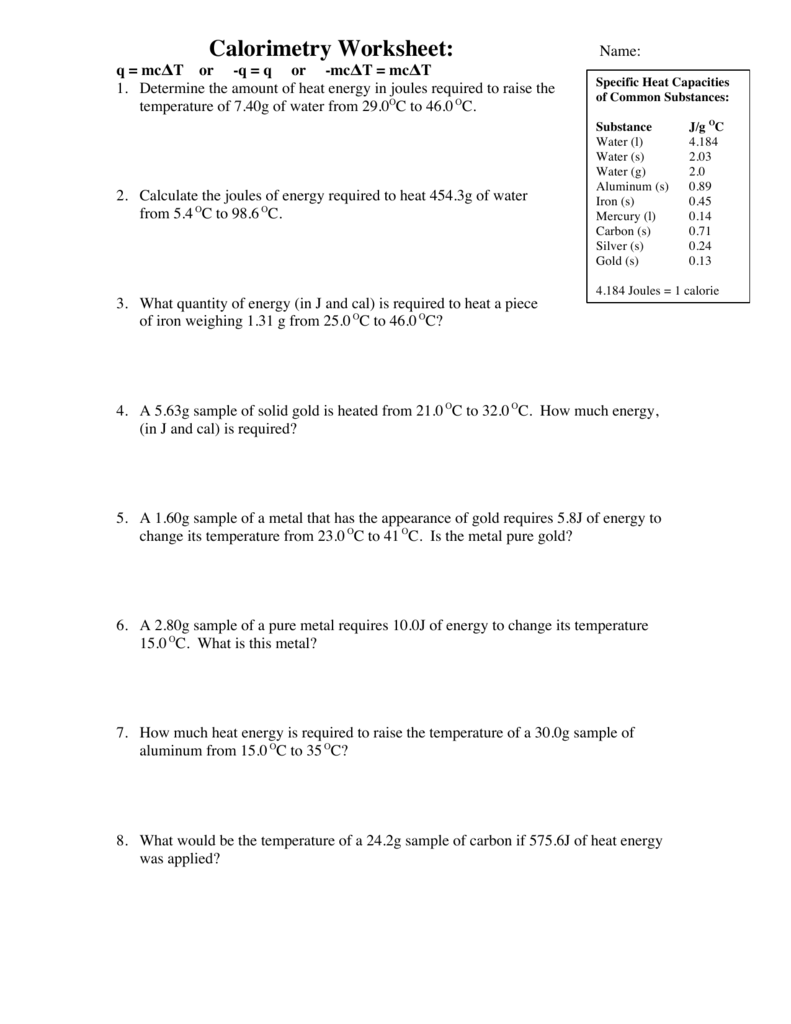
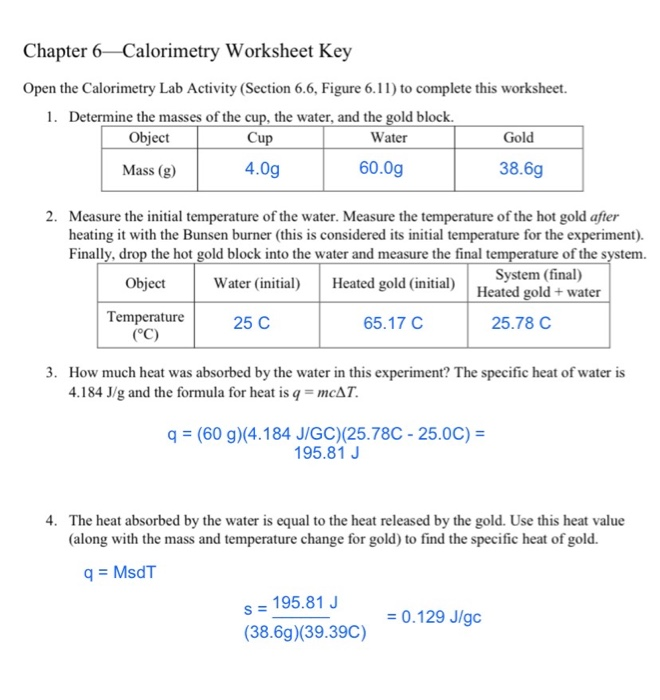
Specific Heat And Calorimetry Worksheet
Specific Heat And Calorimetry Worksheet - How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Web explain the technique of calorimetry. Caloric and joule’s discovery questions: Name:______________________________ pd_____ specific heat and calorimetry (heat lost=heat gained) worksheet 1. One technique we can use to measure the amount of. Web c = specific heat (for water = 4.184 j/goc) 1. Calculate and interpret heat and related properties using typical calorimetry data. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? A calorimeter has a heat capacity of 4.18. Show all work and units. In the table, the first specific heat. One technique we can use to measure the amount of. Name:______________________________ pd_____ specific heat and calorimetry (heat lost=heat gained) worksheet 1. Determine the specific heat of iron if 6.1 j of energy are needed to warm 1.50 of iron from 20.00c to 29.00cs 5. Specific heat, latent heat, phase change graphs, and calorimetry. How much heat is required to raise. Name:______________________________ pd_____ specific heat and calorimetry (heat lost=heat gained) worksheet 1. Web specific heat and calorimetry (heat lost=heat gained) worksheet. Determine the specific heat of iron if 6.1 j of energy are needed to warm 1.50 of iron from 20.00c to 29.00cs 5. Show all work and units. How much heat is required to raise. Use q = (m)(cp))(δt)to solve the following problems. The si unit for specific heat is j. Web which kind of substance needs more energy to undergo an increase of 5 oc, something with a high or low specific heat? One technique we can use to measure the amount of. Web the specific heats of gases depend on what is maintained constant during the heating—typically either the volume or the pressure. In the table, the first specific heat. How much heat is required to raise. Web heat capacity and calorimetry. Endothermic and exothermic energy diagrams entropy. Since specific heat can be. Web specific heat worksheet show all work and proper units. What was the caloric model?. G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; A calorimeter has a heat capacity of 4.18. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Calculate the amounrqofheat (in kj) required to raise the. Specific heat, latent heat, phase change graphs, and calorimetry objective a: What was the caloric model?. One technique we can use to measure the amount of. G iron rod is heated to a temperature t_1 t 1 and then dropped into 20.\; Web c = specific heat (for water = 4.184 j/goc) 1. Calculate the specific heat capacity of the metal. G of water at a lower temperature t_2 t 2 in. Web specific heat worksheet show all work and proper units. Since specific heat can be. Name:______________________________ pd_____ specific heat and calorimetry (heat lost=heat gained) worksheet 1. Calculate the amounrqofheat (in kj) required to raise the. In this worksheet, we will practice performing calorimetry experiments and using the results to calculate the enthalpy. Web specific heat and heat capacity worksheet. Calculate the specific heat capacity of the metal. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Web which kind of substance needs more energy to undergo an increase of 5 oc, something with a high or low specific heat? Heat — — specific heat x mass x temperature 1. Web the specific. Specific heat, latent heat, phase change graphs, and calorimetry objective a: One technique we can use to measure the amount of. Since specific heat can be. A calorimeter has a heat capacity of 4.18. How many grams of water can be heated from 20.0 oc. G of water at a lower temperature t_2 t 2 in. Specific heat, latent heat, phase change graphs, and calorimetry objective a: Use q = (m)(cp))(δt)to solve the following problems. What was the caloric model?. Web which kind of substance needs more energy to undergo an increase of 5 oc, something with a high or low specific heat? One technique we can use to measure the amount of. They cover the following concepts: Calculate the specific heat capacity of the metal. In this worksheet, we will practice performing calorimetry experiments and using the results to calculate the enthalpy. In the table, the first specific heat. Heat — — specific heat x mass x temperature 1. Endothermic and exothermic energy diagrams entropy. Web c = specific heat (for water = 4.184 j/goc) 1. Name:______________________________ pd_____ specific heat and calorimetry (heat lost=heat gained) worksheet 1. Calculate the amounrqofheat (in kj) required to raise the. Web explain the technique of calorimetry. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web calorimetry practice problems 1. Web specific heat and heat capacity worksheet. Caloric and joule’s discovery questions: They cover the following concepts: Endothermic and exothermic energy diagrams entropy. Heat — — specific heat x mass x temperature 1. Web explain the technique of calorimetry. In this worksheet, we will practice performing calorimetry experiments and using the results to calculate the enthalpy. Calculate the amounrqofheat (in kj) required to raise the. One technique we can use to measure the amount of. Since specific heat can be. Show all work and units. What was the caloric model?. Web do you need a worksheet activity to help gather information about student understanding of specific heat and calorimetry? Web the specific heat is numerically equal to the amount of heat necessary to change the temperature of 1.00 k g of mass by 1.00 o c. Web specific heat and calorimetry (heat lost=heat gained) worksheet. How many joules of heat are. G of water at a lower temperature t_2 t 2 in.Calorimetry Worksheet Answer Key
30 Calorimetry Worksheet Answer Key Education Template
Calorimetry Worksheet
Specific Heat Worksheet 2 Answer Key Thekidsworksheet
Calorimetry Problems Worksheet Specific Heat And Calorimetry Packet
Specific Heat Worksheet Answer Key Briefencounters
34 Specific Heat Worksheet Answer Key support worksheet
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Solved Calorimetry Practice Worksheet 1. A small pebble is
specific heat and calorimetry webquest
Web The Specific Heats Of Gases Depend On What Is Maintained Constant During The Heating—Typically Either The Volume Or The Pressure.
How Much Energy Is Needed To Change The Temperature Of 50.0 G Of Water By 15.0Oc?
G Iron Rod Is Heated To A Temperature T_1 T 1 And Then Dropped Into 20.\;
A Calorimeter Has A Heat Capacity Of 4.18.
Related Post: