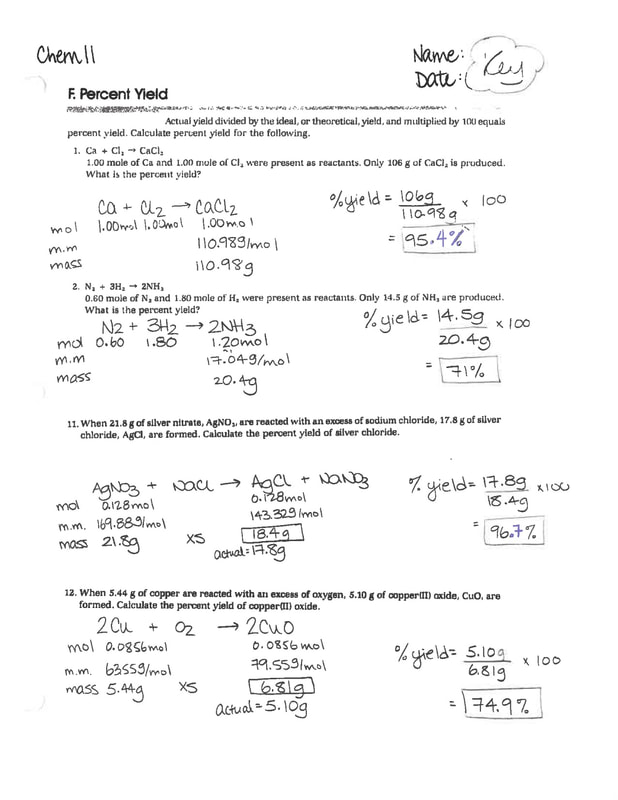
Stoichiometry Worksheet 2 Percent Yield
Stoichiometry Worksheet 2 Percent Yield - Web live worksheets > english > chemistry > stoichiometry > percentage yield exercises. Write the balanced chemical equation. Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. Percent yield =82.74% ~ 83%. Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the. Web theoretical mass of co 2 produced = 13.71 g. First, establish the reaction of c and o2 to produce co2 as shown below. Web stoichiometry and percent yield. This worksheet contains 15 chemical equations for your students to balance along with a stoichiometric calculation. In this lesson, we will learn. Web to do this, start by dividing the smallest number of moles into each of the numbers of moles of elements (i.e., set the smallest number to 1). Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams. Web this worksheet has 10 percent yield stoichiometry problems. First, establish the reaction of c and o2 to produce co2 as shown below. Web stoichiometry and percent yield. Percent yield for each of the problems below: Percent yield =82.74% ~ 83%. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. This worksheet contains 15 chemical equations for your students to balance along with a stoichiometric calculation. Write the balanced chemical equation. Web view stoichiometry worksheet 2 percent yield.pdf from chem 101 at gen douglas macarthur senior high school. Web this worksheet has 10. Write the balanced chemical equation. Identify the given (with units) and what you. Write the balanced chemical equation. Percent yield =82.74% ~ 83%. First, establish the reaction of c and o2 to produce co2 as shown below. Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation, then determine the limiting and excess reactants. 2) according to the following equation, calculate the percentage yield if 550.0 g of toluene (c7h8 )added to an. Write the balanced chemical equation. Web theoretical mass of co 2 produced =. Practice the calculations to find the limiting reagents and yields. First, establish the reaction of c and o2 to produce co2 as shown below. Web up to 24% cash back determine the mass of water vapor you would expect to form (and the percent yield) in the reaction between 15.8 g of nh3 and excess oxygen to produce water and.. Percent yield for each of the problems below: \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Practice the calculations to find the limiting reagents and yields. Identify the given (with units) and what you. Web live worksheets > english > chemistry > stoichiometry > percentage yield exercises. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Web theoretical mass of co 2 produced = 13.71 g. Web the percent yield is calculated as follows: Identify the given (with units) and what you. Practice the calculations to find the limiting reagents and yields. Identify the given (with units) and what you. First, establish the reaction of c and o2 to produce co2 as shown below. 1) 1 n 2 + 3 f 2 2 nf 3 2) 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 3) 1 hbr + 1 khco 3 1 h.. Write the balanced chemical equation. First, establish the reaction of c and o2 to produce co2 as shown below. Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55. Web view stoichiometry worksheet 2 percent yield.pdf from chem 101 at gen douglas macarthur senior high school. Identify the given (with units) and what you. 1) 1 n 2 + 3 f 2 2 nf 3 2) 2 c 6 h 10 + 17 o 2 12 co 2 + 10 h 2 o 3) 1 hbr + 1 khco 3 1 h. Web live worksheets > english > chemistry > stoichiometry > percentage yield exercises. A series of free high school chemistry lessons. Write the balanced chemical equation. Web stoichiometry and percent yield. Percent yield =82.74% ~ 83%. Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the. Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. Web this worksheet has 10 percent yield stoichiometry problems. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. This worksheet contains 15 chemical equations for your students to balance along with a stoichiometric calculation. Web theoretical mass of co 2 produced = 13.71 g. Percent yield for each of the problems below: Web the percent yield is calculated as follows: \text {percent yield} = \dfrac {\text {actual yield}} {\text {theoretical yield}} \times 100\% percent yield = theoretical yieldactual yield ×. Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation, then determine the limiting and excess reactants. Web up to 24% cash back determine the mass of water vapor you would expect to form (and the percent yield) in the reaction between 15.8 g of nh3 and excess oxygen to produce water and. Balanced equations and the starting amount of each reactant are given along with the actual obtained amount of each. 2) according to the following equation, calculate the percentage yield if 550.0 g of toluene (c7h8 )added to an. Web up to 24% cash back determine the mass of water vapor you would expect to form (and the percent yield) in the reaction between 15.8 g of nh3 and excess oxygen to produce water and. This worksheet contains 15 chemical equations for your students to balance along with a stoichiometric calculation. A series of free high school chemistry lessons. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Web c7h6o3 + ___c4h6o3 ( ___c9h8o4 + ___c2h4o2. Web a complete, no prep worksheet that includes 4 problems requiring students to balance or write and balance a chemical equation, then determine the limiting and excess reactants. Identify the given (with units) and what you. In this lesson, we will learn. Web the percent yield is calculated as follows: Percent yield =82.74% ~ 83%. First, establish the reaction of c and o2 to produce co2 as shown below. Web limiting reactant and percentage yield. Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess of all the. Web this worksheet has 10 percent yield stoichiometry problems. Web live worksheets > english > chemistry > stoichiometry > percentage yield exercises.Module 2_Stoichiometry & Percent Yield Stoichiometry Mole (Unit)
Stoichiometry Worksheet 2
stoichiometry worksheet 2 percent yield
Stoichiometry Test worksheet
Limiting Reactant And Percent Yield Worksheet Answers Kidsworksheetfun
Stoichiometry Worksheet 2 Percent Yield Answers Kayra Excel
14 Stoichiometry Worksheet 2 Answer Key /
Stoichiometry Worksheet 2 Percent Yield Answers Tomas Blog
Stoichiometry Problems Chem Worksheet 12 2 Answer Key Worksheet List
Stoichiometry Worksheet 2 Percent Yield worksheet
The Amount Of Product That May Be Produced By A Reaction Under Specified Conditions, As Calculated Per The Stoichiometry Of An Appropriate Balanced Chemical.
Write The Balanced Chemical Equation.
1) 1 N 2 + 3 F 2 2 Nf 3 2) 2 C 6 H 10 + 17 O 2 12 Co 2 + 10 H 2 O 3) 1 Hbr + 1 Khco 3 1 H.
Balanced Equations And The Starting Amount Of Each Reactant Are Given Along With The Actual Obtained Amount Of Each.
Related Post: