The Gas Laws Worksheet Answers
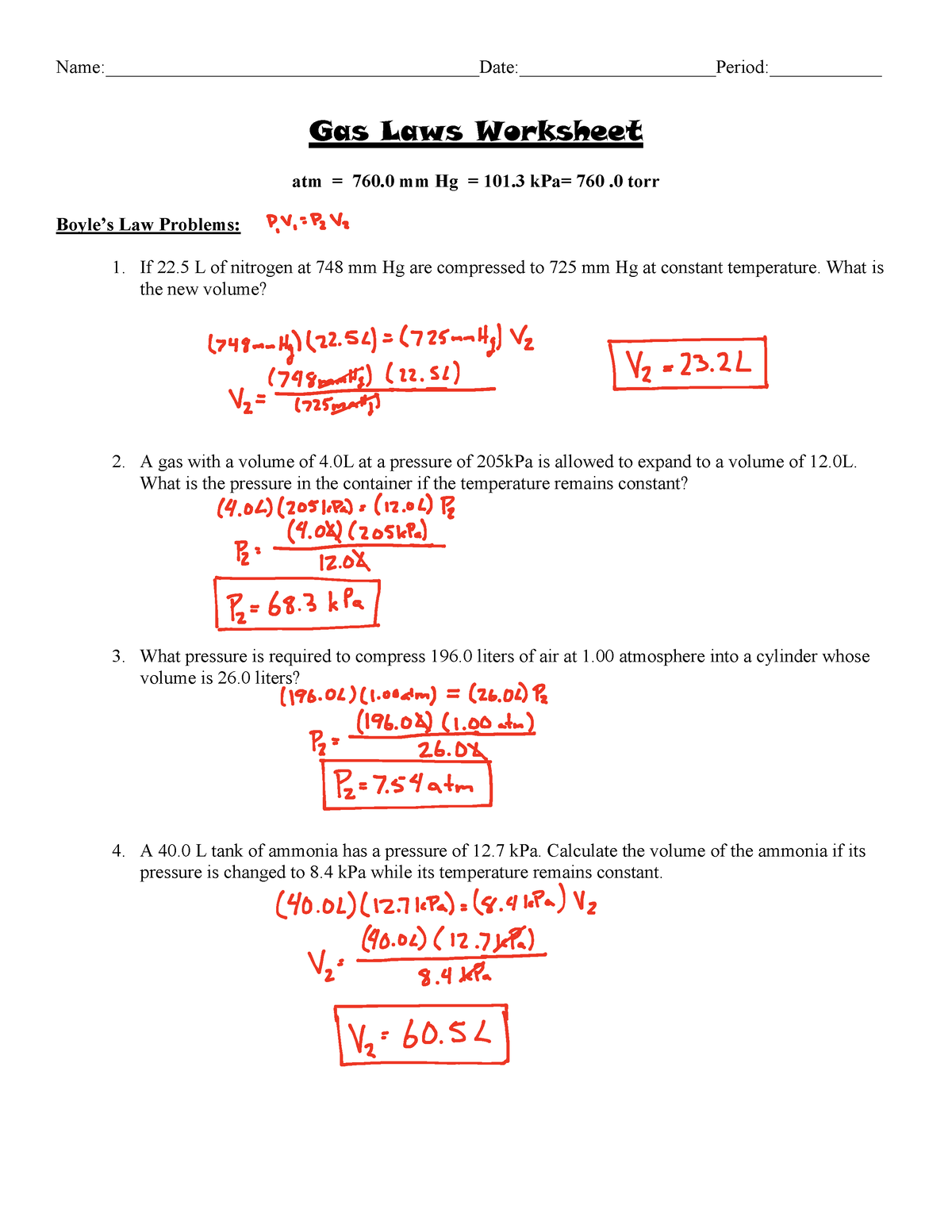
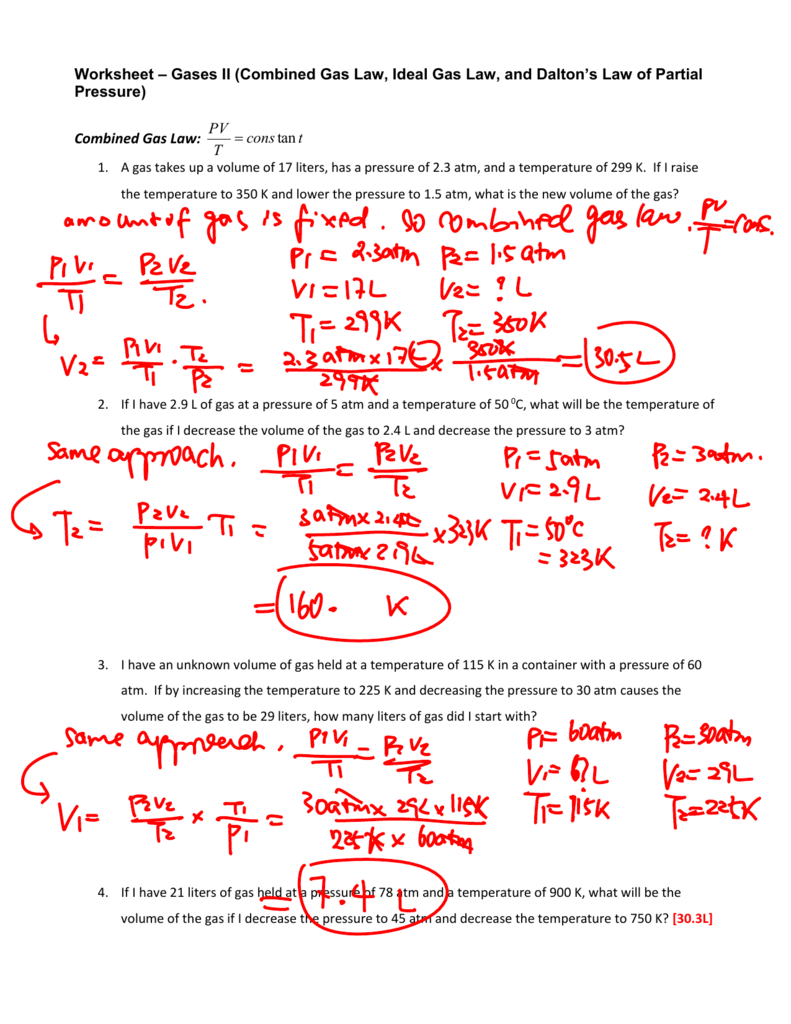
The Gas Laws Worksheet Answers - If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? What is the new volume? If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Here are the links to the topics covered. The following practice problems are to master to topics on the ideal gas laws: The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. The gas pressure if the temperature is decreased (at constant v)? Where p =pressure, v = volume, n = number of moles of gas (amount of gas), r = gas constant, t = temperature. Web consider a sample of gas in a cylinder with a moveable piston. 1 atm = 760.0 mm hg = 101.3 kpa. The gas pressure if the temperature is decreased (at constant v)? A gas with a volume of 4 at a pressure of 205kpa is allowed to expand to a volume of 12. 1973 k 2) if i initially have a gas with a pressure of 84 kpa and a temperature of 350 The following practice problems are to master to. There are 12 problems total.answer key is include. 1 atm = 760.0 mm hg = 101.3 kpa. If 22.5 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Assuming that no gas is lost or gained (constant n), what would happen to: There are examples to work on the dalton law of partial. What is the new volume? Volume, pressure, temperature, number of moles, and the ideal gas constant are covered in 18 unique gas laws worksheets. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Since the amount of gas n (in the system) and the temperature t remain unchanged, the right side of the equation remains constant. There are 12. Atm = 760 mm hg = 101 kpa= 760.0 torr. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. Assuming that no gas is lost or gained (constant n), what would happen to: Web consider a sample of gas in a cylinder with a moveable piston. The gas volume if the temperature is increased (at constant p)? Each of these laws can be derived from this law. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Assuming that no gas is lost or gained (constant n), what would happen to: If it involves moles or grams, it must be pv = nrt 1) if four. What is the new volume? What is the pressure in the container if the temperature. The gas pressure if the temperature is decreased (at constant v)? Web use your knowledge of the ideal and combined gas laws to solve the following problems. Atm = 760 mm hg = 101 kpa= 760.0 torr. There are 12 problems total.answer key is include. What is the pressure in the container if the temperature. What is the new volume? What is the new volume? Each of these laws can be derived from this law. Web use your knowledge of the ideal and combined gas laws to solve the following problems. Here are the links to the topics covered. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? What. Web consider a sample of gas in a cylinder with a moveable piston. Atm = 760 mm hg = 101 kpa= 760.0 torr. Since the amount of gas n (in the system) and the temperature t remain unchanged, the right side of the equation remains constant. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law. 1 atm = 760.0 mm hg = 101.3 kpa. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law equation. Web use your knowledge of the ideal and combined gas laws to solve the following problems. This product includes worksheet, answer key, instruction, and other activity. Web gas laws crossword puzzle and answer key this crossword. The gas pressure if the temperature is decreased (at constant v)? The following practice problems are to master to topics on the ideal gas laws: Where p =pressure, v = volume, n = number of moles of gas (amount of gas), r = gas constant, t = temperature. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law equation. Volume, pressure, temperature, number of moles, and the ideal gas constant are covered in 18 unique gas laws worksheets. If it involves moles or grams, it must be pv = nrt 1) if four moles of a gas at a pressure of 5.4 atmospheres have a volume of 120 liters, what is the temperature? There are examples to work on the dalton law of partial pressures, the graham’s law of effusion, and gas stoichiometry. Since the amount of gas n (in the system) and the temperature t remain unchanged, the right side of the equation remains constant. The gas volume if the temperature is increased (at constant p)? Web consider a sample of gas in a cylinder with a moveable piston. The gas volume if the pressure is increased (at constant t)? Here are the links to the topics covered. What is the new volume? Each of these laws can be derived from this law. A gas with a volume of 4 at a pressure of 205kpa is allowed to expand to a volume of 12. What is the new volume? Web use your knowledge of the ideal and combined gas laws to solve the following problems. A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Web gas laws crossword puzzle and answer key this crossword puzzle contains vocabulary pertaining to the gas laws and the properties of gases. Atm = 760 mm hg = 101 kpa= 760.0 torr. The gases in a hair spray can are at a temperature of 27 oc and a pressure of 30 lbs/in2. What is the new volume? The gas volume if the pressure is increased (at constant t)? There are examples to work on the dalton law of partial pressures, the graham’s law of effusion, and gas stoichiometry. Web use your knowledge of the ideal and combined gas laws to solve the following problems. Web gas laws 1 (worksheet) formulas & masses (worksheets) solutions. If 22 l of nitrogen at 748 mm hg are compressed to 725 mm hg at constant temperature. Since the amount of gas n (in the system) and the temperature t remain unchanged, the right side of the equation remains constant. A gas with a volume of 4 at a pressure of 205kpa is allowed to expand to a volume of 12. Volume, pressure, temperature, number of moles, and the ideal gas constant are covered in 18 unique gas laws worksheets. What is the new volume? A gas with a volume of 4.0l at a pressure of 205kpa is allowed to expand to a volume of 12.0l. Boyle’s law, charles’s law, and avogadro’s law, as well as the combined gas law equation. Web consider a sample of gas in a cylinder with a moveable piston. The gas pressure if the temperature is decreased (at constant v)? N = pv = (2.8 atm)(98 l) = 11 moles of gas rt (0.0821 l.atm/mol.k)(292 k) 2) if 5.0 moles of o 2 and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 0Mixed Gas Law Worksheet Answers available at end Name (show
Gas Laws Worksheet III Answer Key 1112 Gases Mole (Unit)
Chemistry Gas Laws Worksheet Answers / Ideal Gas Law Worksheet Pv Nrt
Gas Laws Worksheet answer key Gas Laws Worksheet atm = 760 mm Hg
Pin di Worksheet
Combined Gas Law Worksheet Answers
Gas Law Worksheet Answers Free Download Goodimg.co
17 Mixed Gas Laws Worksheet Answers /
Combined Gas Law Worksheet Answer Key —
Worksheet Gas Laws II Answers
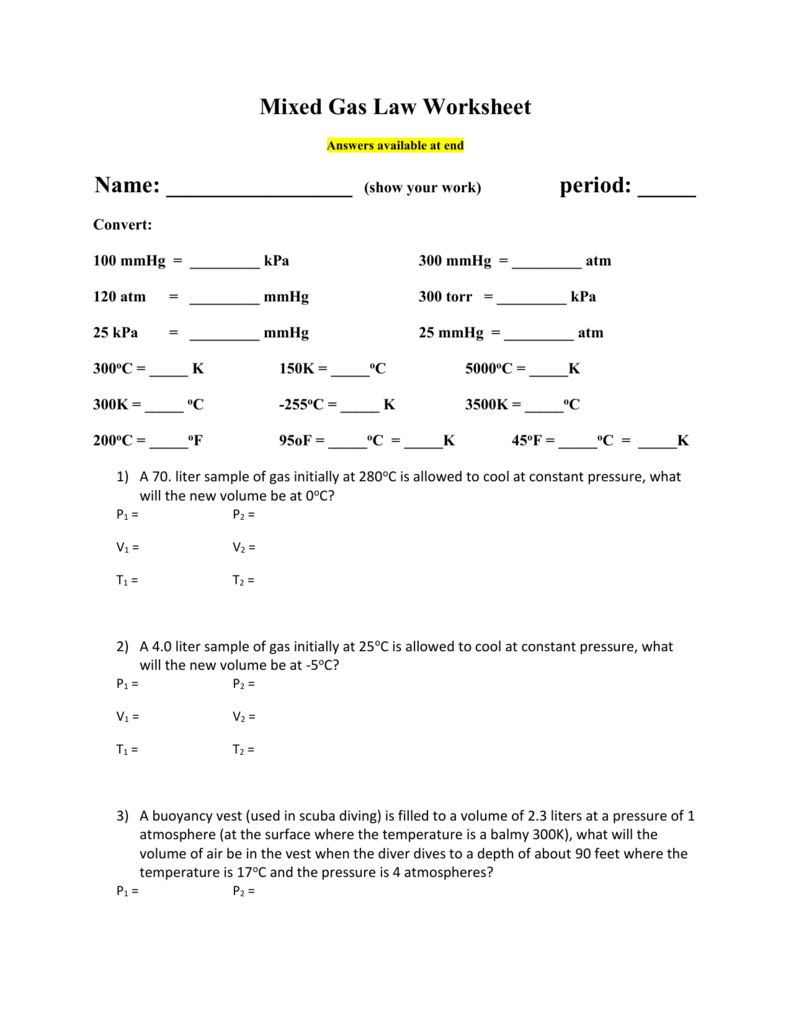
1 Atm = 760.0 Mm Hg = 101.3 Kpa.
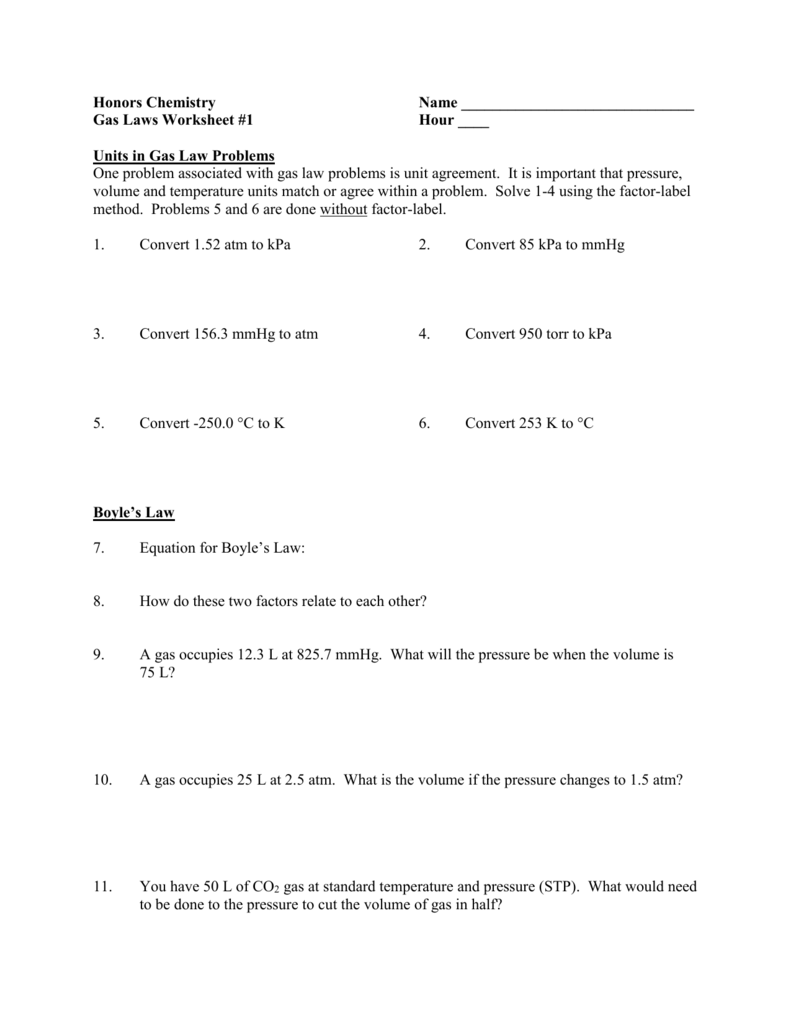
Here Are The Links To The Topics Covered.
If The Gases In The Can Reach A Pressure Of 90 Lbs/In2, The Can Will Explode.
There Are 12 Problems Total.answer Key Is Include.
Related Post: