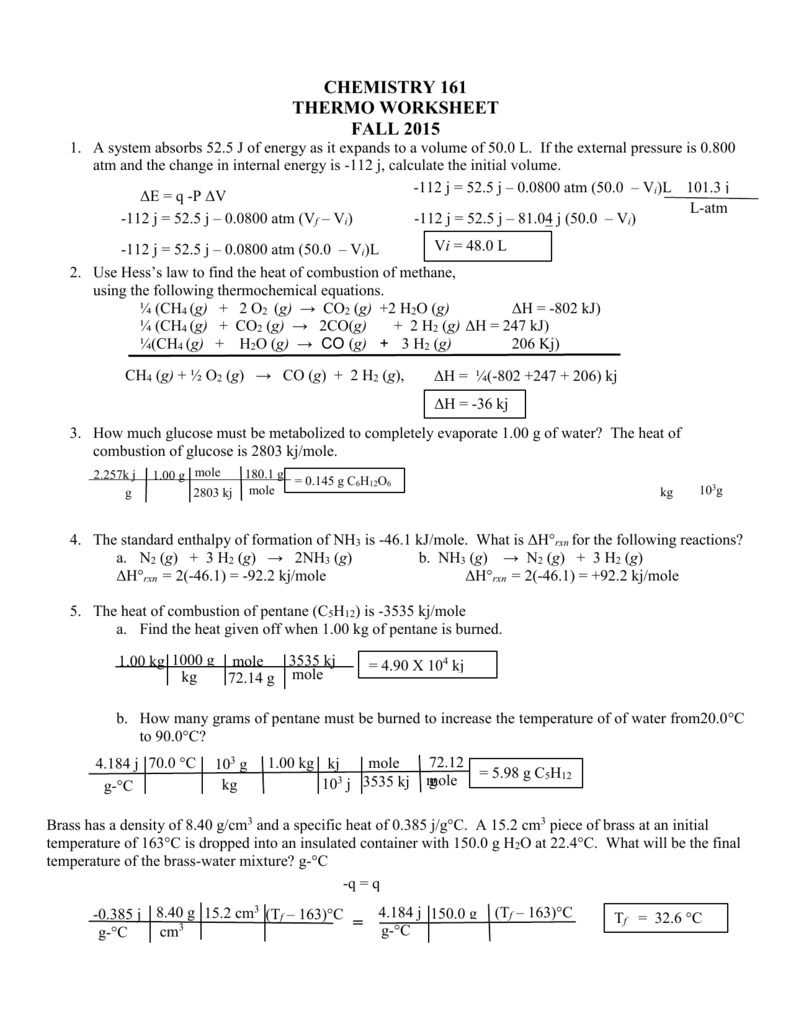
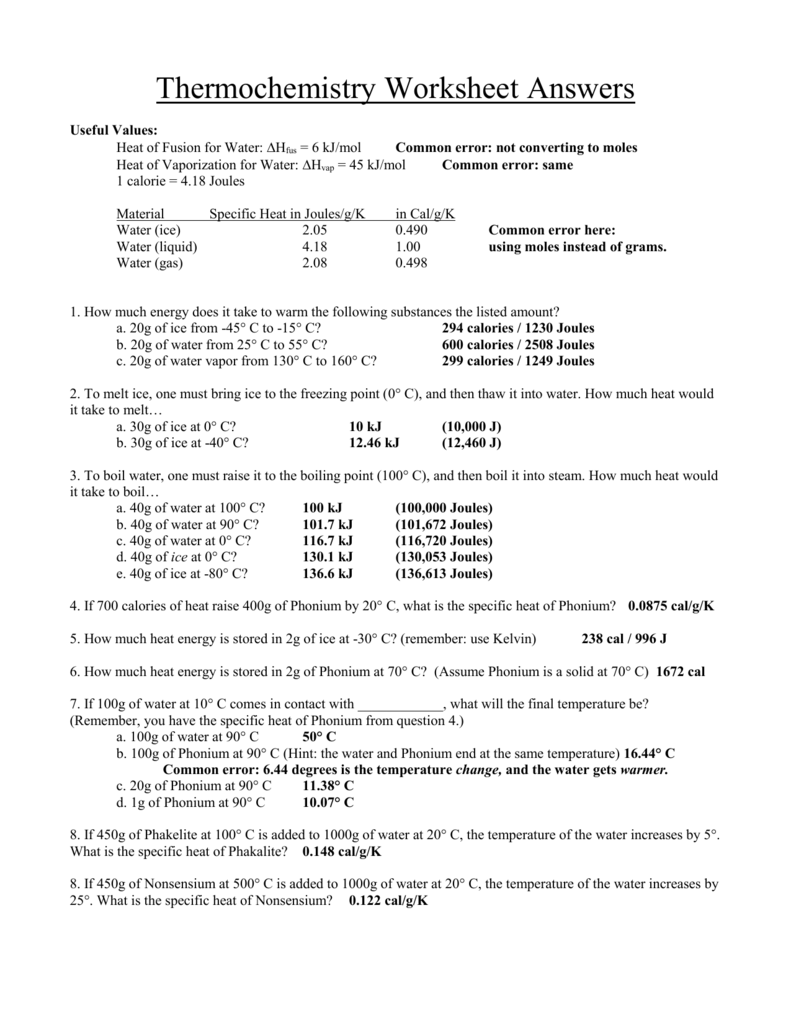
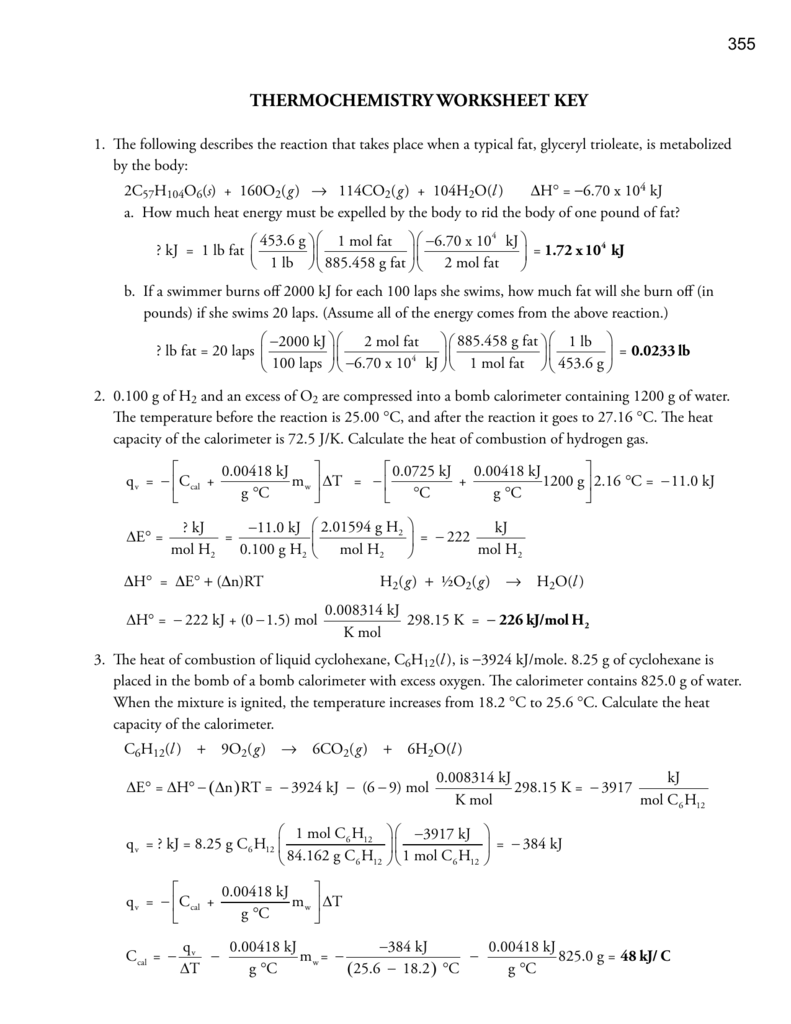
Thermochemistry Worksheet 1 Answer Key
Thermochemistry Worksheet 1 Answer Key - Web for the following reaction: Web the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. What is ∆h for the reverse reaction? Describe terms for phase changes. More practice with calorimetry problemsws3:. Download this workbook which contains full solutions: The three variables are related by the equation. Thermochemical equations, rate laws, bond enthalpy, pe diagrams, and much more. Web short answer / problems: How much heat energy must be expelled by the body to rid the body of one pound of fat? The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: 1) (50.0 g)(15.0 °c) (2.06 j/g °c) heating of a solid = 1540 j (3 significant digits. A) pcl 3(l) + cl 2(g) pcl 5(g) + energy b) c (s) + h 2 o (g) co (g) + h 2(g) Web. Describe terms for phase changes. (a) the solid to liquid state. Download this workbook which contains full solutions: What is ∆h for the reverse reaction? (b) the gas to the liquid state. 6 worksheets (including the answer keys for each) on topics in. The value of c in this equation, and likewise the magnitudes of q and δ t, pertain to a certain sample and depend on the amount. (a) the solid to liquid state. What is ∆h when 20.0g of s 8 reacts? More practice with calorimetry problemsws3:. 1) (50.0 g)(15.0 °c) (2.06 j/g °c) heating of a solid = 1540 j (3 significant digits. What is ∆h when 20.0g of s 8 reacts? Web the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. A) pcl 3(l) + cl 2(g) pcl 5(g) +. Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. Web thermochemistry worksheets teaching resources. 6 worksheets (including the answer keys for each) on topics in. Web for high school thermochemistry step 1: Is this an exothermic or endothermic reaction? Thermochemical equations, rate laws, bond enthalpy, pe diagrams, and much more. Save or instantly send your ready documents. 1) (50.0 g)(15.0 °c) (2.06 j/g °c) heating of a solid = 1540 j (3 significant digits. Web for the following reaction: Describe terms for phase changes. ∆hfus = 206 j/g, ∆hvap = 4 kj/g. The three variables are related by the equation. A) pcl 3(l) + cl 2(g) pcl 5(g) + energy b) c (s) + h 2 o (g) co (g) + h 2(g) 6 worksheets (including the answer keys for each) on topics in. Worksheet thermochemistry ii answers 1 what is happening to the Thermochemistry answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. Web for the following reaction: How much heat, in calories, is released or absorbed if a 15 g sample of copper is converted from. Describe terms for phase changes. A complete answer key is provided at the end. Web complete unit thermochemistry intro and joule conversions wksh 1 answer key online with us legal forms. The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: Save or instantly send your ready documents. Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work. Web thermochemistry worksheet key 1. Web thermochemistry worksheets teaching resources. What is ∆h when 3.2 mol of s 8 reacts? 2) classify each of these reactions as exothermic or endothermic: Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. (b) the gas to the liquid state. 2) classify each of these reactions as exothermic or endothermic: The three variables are related by the equation. 1) distinguish between endothermic and exothermic processes. 1) (50.0 g)(15.0 °c) (2.06 j/g °c) heating of a solid = 1540 j (3 significant digits. Is this an exothermic or endothermic reaction? Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. How much heat, in calories, is released or absorbed if a 15 g sample of copper is converted from. Easily fill out pdf blank, edit, and sign them. Thermochemical equations, rate laws, bond enthalpy, pe diagrams, and much more. Web for the following reaction: The following describes the reaction that takes place when a typical fat, glyceryl trioleate, is metabolized by the body: What is ∆h when 3.2 mol of s 8 reacts? The reaction of magnesium with sulfuric acid was carried out in a calorimeter. Download this workbook which contains full solutions: The value of c in this equation, and likewise the magnitudes of q and δ t, pertain to a certain sample and depend on the amount. Web thermochemistry worksheet 1 answer key this reaction caused the temperature of 270 grams of liquid water within the calorimeter to raise from 250c to 760c. Thermochemistry answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. A) pcl 3(l) + cl 2(g) pcl 5(g) + energy b) c (s) + h 2 o (g) co (g) + h 2(g) (a) the solid to liquid state. Some of the worksheets for this concept are thermochemistry, thermochemistry, thermochemistrypractice thermochemical equations and, thermochemistry calculations work 1, ap chemistry review work unit 4, answers thermochemistry practice problems. Web short answer / problems: ∆hfus = 206 j/g, ∆hvap = 4 kj/g. The reaction of magnesium with sulfuric acid was carried out in a calorimeter. Easily fill out pdf blank, edit, and sign them. Thermochemistry answers worksheet number one we will ignore any heats losses to the walls of the container and losses to the air. A) pcl 3(l) + cl 2(g) pcl 5(g) + energy b) c (s) + h 2 o (g) co (g) + h 2(g) Give at least 3 examples of each that you encounter in everyday life. How much heat, in calories, is released or absorbed if a 15 g sample of copper is converted from. More practice with calorimetry problemsws3:. Web the heat capacity, c, is the amount of heat, q, required to raise the temperature, δ t, of an object by 1 o c. A complete answer key is provided at the end. 6 worksheets (including the answer keys for each) on topics in. Web for the following reaction: (b) the gas to the liquid state. What is ∆h when 3.2 mol of s 8 reacts?enthalpy worksheet with answers
Thermochemistry worksheet 1 Key
enthalpy stoichiometry worksheet
Thermochemistry Worksheet 1 Answers Promotiontablecovers
Thermochemistry Worksheet
Thermochemistry Calorimetry Worksheet Answers Reporteral
Thermochemistry Review Worksheet Key kidsworksheetfun
Thermochemistry Practice Sheet Answer Key Properties Of Water Heat
Thermochemistry Worksheet 1 Answers Promotiontablecovers
thermochemistry worksheet key
Describe Terms For Phase Changes.
What Is ∆H For The Reverse Reaction?
Web Thermochemistry Worksheets Teaching Resources.
Web Thermochemistry Worksheet 1 Answer Key This Reaction Caused The Temperature Of 270 Grams Of Liquid Water Within The Calorimeter To Raise From 250C To 760C.
Related Post: