Unit Periodic Trends Ionization Energy Trend Worksheet 3 Answer Key
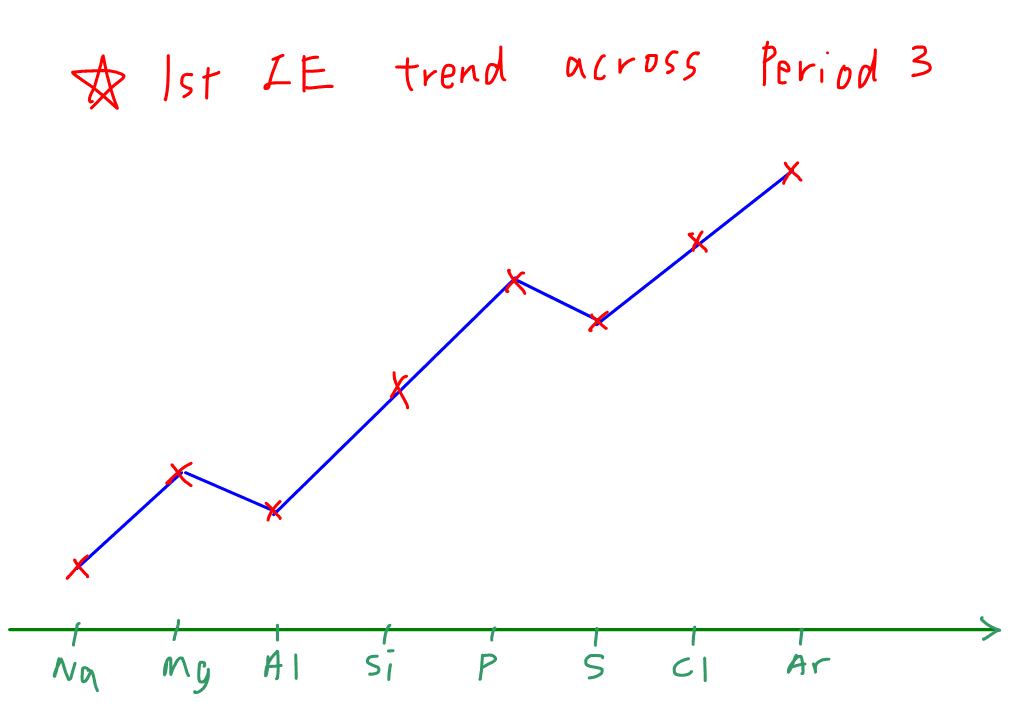
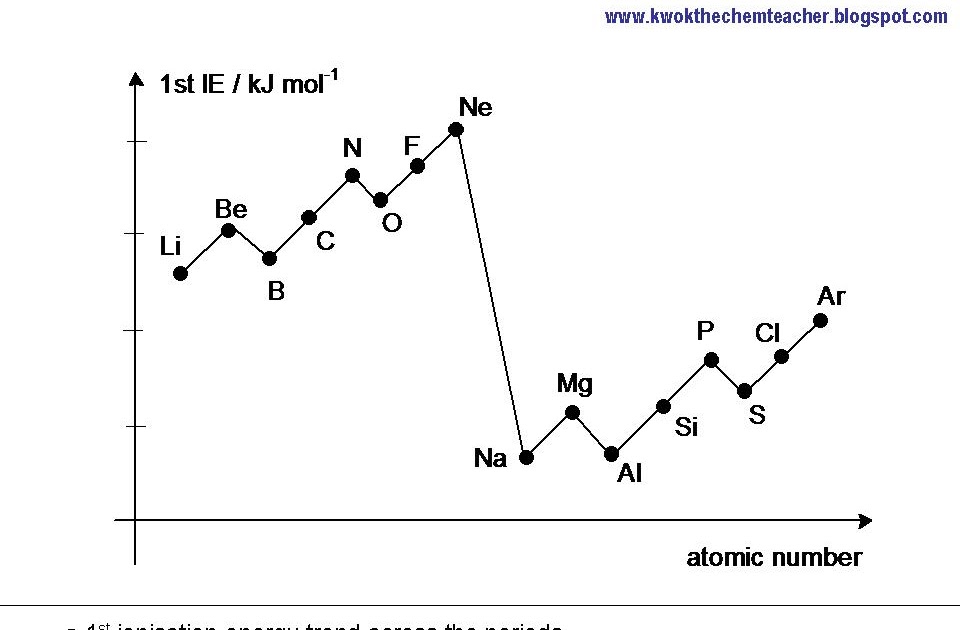
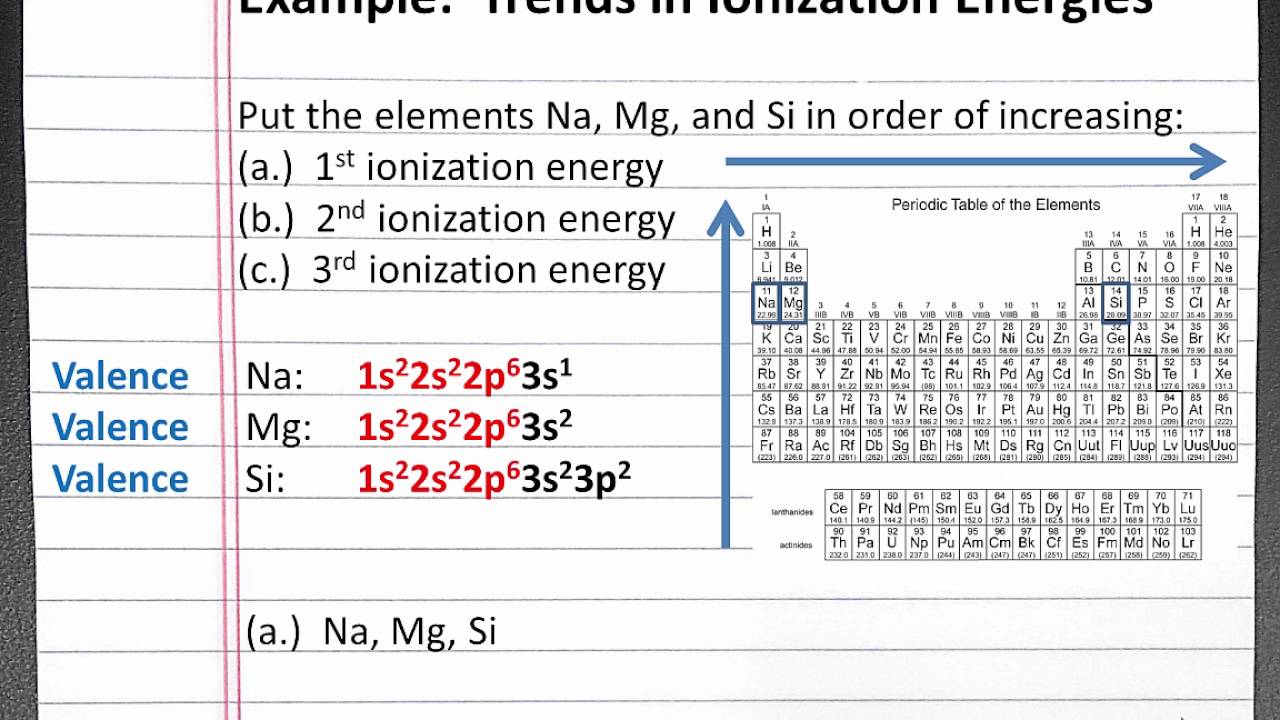
Unit Periodic Trends Ionization Energy Trend Worksheet 3 Answer Key - It is more difficult to remove a second electron from an atom. Energy + x (g) → x+ (g). The higher the ionization energy, the more difficult it is to remove the electron. Why is the second ionization energy greater than the first ionization energy? Web as elements of group 1 of the periodic table are considered in order from top to bottom, the ionization energy of each successive element decreases. What trend do you notice? Describe the factors that affect the energy. It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. What is the ionization energy of an element? Please answer each fill in the blank with the best answer. Sap‑1 (eu), sap‑1.a (lo), sap‑1.a.4 (ek), sap‑2 (eu), sap‑2.a (lo), sap‑2.a.2 (ek), sap‑2.a.3 (ek) google classroom. Energy + x (g) → x+ (g). Web lose electrons when they form ions. Ionization energy tends to decrease down a group. Ionization energy, electronegativity, and the relative sizes of ions and atoms (this includes knowing. Gather data for ionization energy across a period. Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization. Describe the factors that affect the energy. Please answer each fill in the blank with the best answer. This bundle of 5 homework assignments includes questions that reviews. Describe the factors that affect the energy. Students know how to use the periodic table to identify the following trends: What is the ionization energy of an element? The higher the ionization energy, the more difficult it is to remove the electron. Web the first ionization energy of beryllium is 9.322 ev, the second ionization energy is 18.211 ev, and. What trend do you notice? Explain why the third ionization. Web lose electrons when they form ions. It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. What is the ionization energy of an element? Ionization energy, electronegativity, and the relative sizes of ions and atoms (this includes knowing. Web lose electrons when they form ions. It is more difficult to remove a second electron from an atom. Web the trend for ionization energy as you go across a period is that ionization energy increases b/c of the increase in protons, and the atomic size. Web the first ionization energy of beryllium is 9.322 ev, the second ionization energy is 18.211 ev, and the third ionization energy is 153.893 ev. Ionization energy tends to decrease down a group. Describe the factors that affect the energy. Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization.. This decrease is due to. Describe the factors that affect the energy. Web the first ionization energy of beryllium is 9.322 ev, the second ionization energy is 18.211 ev, and the third ionization energy is 153.893 ev. It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. (the. Why is the second ionization energy greater than the first ionization energy? The higher the ionization energy, the more difficult it is to remove the electron. Ionization energy tends to decrease down a group. This bundle of 5 homework assignments includes questions that reviews. Gather data for ionization energy across a period. What trend do you notice? It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. (the energy required to remove an electron from a. Web use the concept of ionization energy to explain why sodium form a 1+ ion (na+) but magnesium forms a 2+ ion (mg2+) sodium. Gather data for ionization energy across a period. What is the ionization energy of an element? Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization. Sap‑1 (eu), sap‑1.a (lo), sap‑1.a.4 (ek), sap‑2 (eu), sap‑2.a (lo), sap‑2.a.2 (ek), sap‑2.a.3 (ek) google classroom. Web as elements of group 1 of the. The higher the ionization energy, the more difficult it is to remove the electron. It is more difficult to remove a second electron from an atom. Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization. Ionization energy, electronegativity, and the relative sizes of ions and atoms (this includes knowing. What is the ionization energy of an element? This bundle of 5 homework assignments includes questions that reviews. Describe the factors that affect the energy. Web the trend for ionization energy as you go across a period is that ionization energy increases b/c of the increase in protons, and the atomic size decreases, therefore. It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization. Sap‑1 (eu), sap‑1.a (lo), sap‑1.a.4 (ek), sap‑2 (eu), sap‑2.a (lo), sap‑2.a.2 (ek), sap‑2.a.3 (ek) google classroom. Please answer each fill in the blank with the best answer. Web lose electrons when they form ions. Energy + x (g) → x+ (g). This decrease is due to. Web the first ionization energy of beryllium is 9.322 ev, the second ionization energy is 18.211 ev, and the third ionization energy is 153.893 ev. Web as elements of group 1 of the periodic table are considered in order from top to bottom, the ionization energy of each successive element decreases. Ionization energy tends to decrease down a group. (the energy required to remove an electron from a. Web use the concept of ionization energy to explain why sodium form a 1+ ion (na+) but magnesium forms a 2+ ion (mg2+) sodium has a low 1st ionization energy because it. Web unit periodic trends ionization energy trend worksheet 3 answer key the goals of learning determine the energy of ionization. This decrease is due to. Web the trend for ionization energy as you go across a period is that ionization energy increases b/c of the increase in protons, and the atomic size decreases, therefore. What trend do you notice? Describe the factors that affect the energy. Ionization energy, electronegativity, and the relative sizes of ions and atoms (this includes knowing. This bundle of 5 homework assignments includes questions that reviews. It is the minimal energy needed to remove an electron from the outer shell of a neutral atom in a gaseous state. What is the ionization energy of an element? It is more difficult to remove a second electron from an atom. Web lose electrons when they form ions. Why is the second ionization energy greater than the first ionization energy? Web the first ionization energy of beryllium is 9.322 ev, the second ionization energy is 18.211 ev, and the third ionization energy is 153.893 ev. Gather data for ionization energy across a period. Web as elements of group 1 of the periodic table are considered in order from top to bottom, the ionization energy of each successive element decreases. Energy + x (g) → x+ (g).What Is The Equation Ionisation Energy Tessshebaylo
1 Periodic Trends Worksheet (advanced) Chemistry LibreTexts
First And Second Ionization Energy The Periodic Table Variations Of
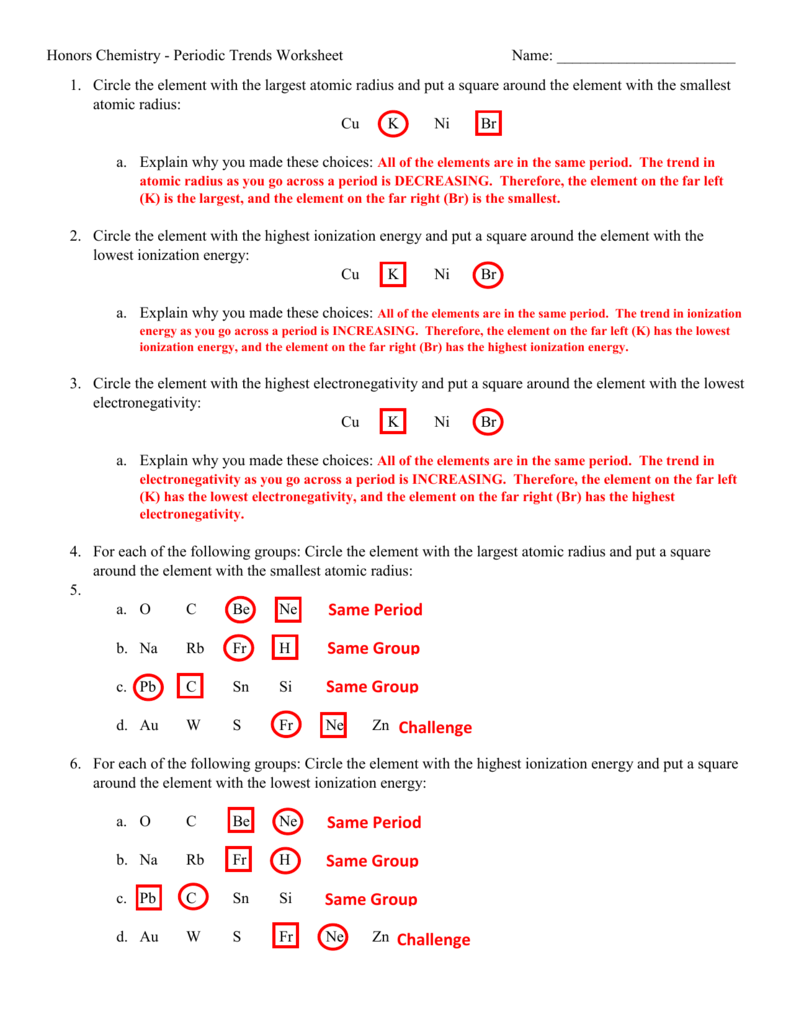
Periodic Trends Worksheet Answer Key
KWOK The Chem Teacher Periodicity Ionisation Energy Trend
Second Ionization Energy Trend Periodic Table Review Home Decor
1st Ionization Energy Table Images Amashusho
Quiz & Worksheet Ionization Energy
Trends in First Ionization Energy Ionization energy, Chemistry class
Periodicity Trends in the Periodic Table Chemistry classroom
(The Energy Required To Remove An Electron From A.
Sap‑1 (Eu), Sap‑1.A (Lo), Sap‑1.A.4 (Ek), Sap‑2 (Eu), Sap‑2.A (Lo), Sap‑2.A.2 (Ek), Sap‑2.A.3 (Ek) Google Classroom.
Describe The Factors That Affect The Energy.
Please Answer Each Fill In The Blank With The Best Answer.
Related Post: