Valence Electrons And Ions Worksheet Answer Key
Valence Electrons And Ions Worksheet Answer Key - ~ identify the element ~ understanding valence. Ions, ion notation, anions, cations, bohr model, oxidation number, ionic compounds. Web valence electrons and ions quiz for 9th grade students. (octet rule, halide ions, chloride ion, electron dot structure, cations,valence electron, anions) valence. Web in the first table, for each of the ions, indicate the total number of protons and electrons. Web key term ions worksheet answer key. Carbon is able to bond with atoms of other elements in many. Web sublevels is elght, there can be no more than eight valence electrons. •valence electrons are the only electrons generally involved in bond. Web it loses 3 valence electrons to form an ion. Web an electron in the highest occupied energy level of an element's atom. Web it loses 3 valence electrons to form an ion. Web key term ions worksheet answer key. A set of worksheets reinforcing how to determine valence electrons, draw lewis dot symbols, determine basic charges, and the number of. When given the protons and electrons, indicate the ion. Respond to the questions and. A set of worksheets reinforcing how to determine valence electrons, draw lewis dot symbols, determine basic charges, and the number of. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. •valence electrons are the only electrons generally involved in bond. Web key term ions worksheet answer. Answer key ions worksheet element # valence electrons # electrons. Respond to the questions and. Ions, ion notation, anions, cations, bohr model, oxidation number, ionic compounds. Find other quizzes for chemistry and more on quizizz for free! When given the protons and electrons, indicate the ion with the correct charge. In the second table, when given the protons and electrons, indicate the ion with the correct. It gains 5 valence electrons to form an ion. Determine the number of valence electrons in the atoms below. ~ identify the element ~ understanding valence. Web pure substances that are created when two or more atoms of different elements react by gaining, losing,. Valence electrons are lost or gained when forming ions so that they have an outermost. Web sublevels is elght, there can be no more than eight valence electrons. Prompts in the orange boxes. Web an electron in the highest occupied energy level of an element's atom. Carbon is able to bond with atoms of other elements in many. Prompts in the orange boxes. Web for each of the following ions, indicate the total number of protons and electrons. Valence electrons are lost or gained when forming ions so that they have an outermost. Web pure substances that are created when two or more atoms of different elements react by gaining, losing, or sharing valence electrons to reach a. Determine the total number of valence (outer shell) electrons. Web key term ions worksheet answer key. Answer key ions worksheet element # valence electrons # electrons. Determine the number of valence electrons in the atoms below. Web i have included a student response document and an answer key with suggested answers. Web key term ions worksheet answer key. This preview shows page 1 out of 1 page. Grade 8 bonds and mixing. Web an electron in the highest occupied energy level of an element's atom. Web let us determine the lewis structures of sih 4, cho 2 −, no +, and of 2 as examples in following this procedure: When given the protons and electrons, indicate the ion with the correct charge. Valence electrons are the s and p electrons in the outermost shell. Determine the number of valence electrons in the atoms below. Web sublevels is elght, there can be no more than eight valence electrons. Web pure substances that are created when two or more atoms of. Determine the number of valence electrons in the atoms below. Web it loses 3 valence electrons to form an ion. Ions, ion notation, anions, cations, bohr model, oxidation number, ionic compounds. Follow the instructions to go through the simulation. Valence electrons are the s and p electrons in the outermost shell. Valence electrons are the s and p electrons in the outermost shell. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. Web for each of the following ions, indicate the total number of protons and electrons. It gains 5 valence electrons to form an ion. Follow the instructions to go through the simulation. Valence electrons are lost or gained when forming ions so that they have an outermost. Determine the total number of valence (outer shell) electrons. Answer key ions worksheet element # valence electrons # electrons. Carbon is able to bond with atoms of other elements in many. Web an electron in the highest occupied energy level of an element's atom. Web i have included a student response document and an answer key with suggested answers. Web as you have learned, ions are atoms or molecules bearing an electrical charge. Web sublevels is elght, there can be no more than eight valence electrons. •valence electrons are the electrons in the highest occupied energy level of the atom. A set of worksheets reinforcing how to determine valence electrons, draw lewis dot symbols, determine basic charges, and the number of. Respond to the questions and. ~ identify the element ~ understanding valence. Web it loses 3 valence electrons to form an ion. Web key term ions worksheet answer key. Included in the chemistry instructor resources subscription. You can do the exercises online or download the worksheet as pdf. Determine the number of valence electrons in the atoms below. A set of worksheets reinforcing how to determine valence electrons, draw lewis dot symbols, determine basic charges, and the number of. This preview shows page 1 out of 1 page. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence. •valence electrons are the only electrons generally involved in bond. Web valence electrons and ions quiz for 9th grade students. Web as you have learned, ions are atoms or molecules bearing an electrical charge. When given the protons and electrons, indicate the ion with the correct charge. Grade 8 bonds and mixing. Find other quizzes for chemistry and more on quizizz for free! Included in the chemistry instructor resources subscription. Web it loses 3 valence electrons to form an ion. •valence electrons are the electrons in the highest occupied energy level of the atom. ~ identify the element ~ understanding valence. Web pure substances that are created when two or more atoms of different elements react by gaining, losing, or sharing valence electrons to reach a stable electron arrangement.Valence Electrons and Lewis Dot Structure Worksheet Answers Worksheet
[EXCLUSIVE] Valence Electrons Chart Worksheet Answers
Valence Electrons Worksheet Answer Key worksheet
valence electron worksheet answers
Valence Electrons Worksheet Free Worksheets Samples
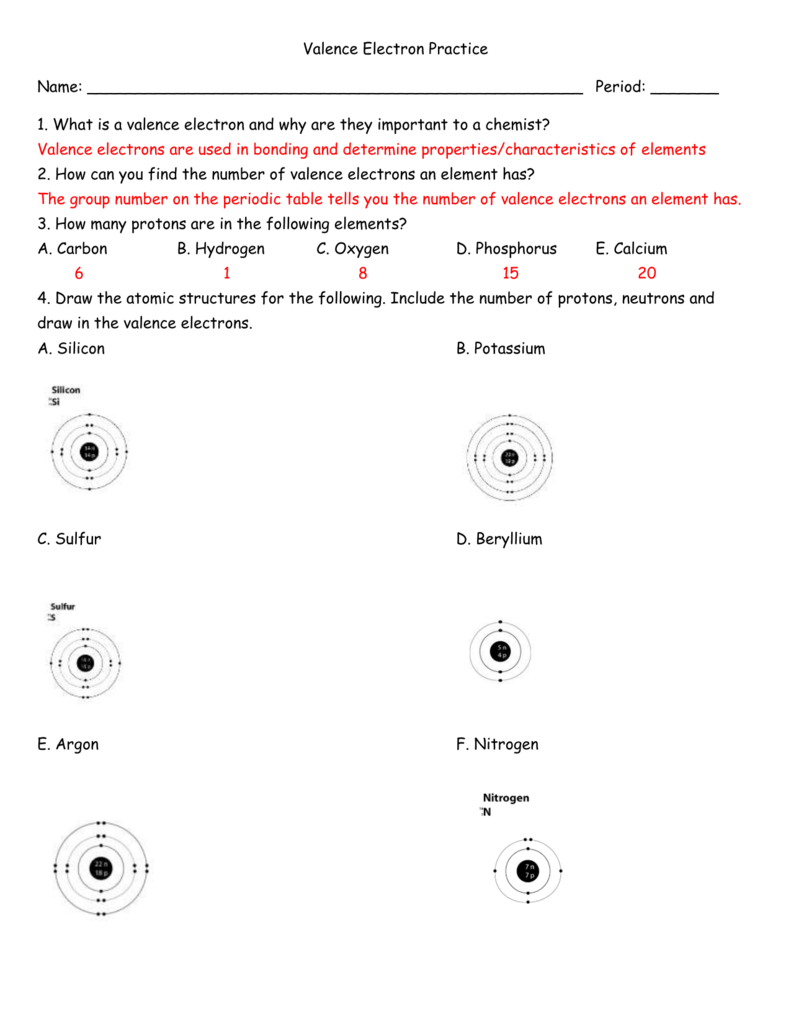
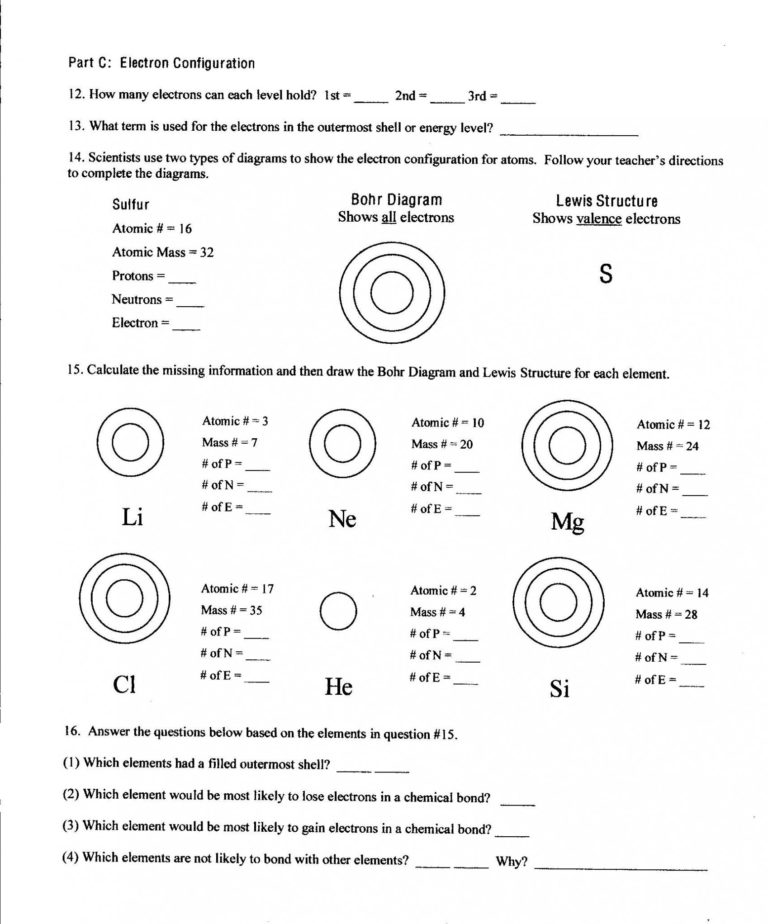
Valence Electron Practice Worksheet Answers
Worksheet Chemical Bonding Ionic And Covalent Answers Part 2 —
Counting atoms Worksheet Answer Key Education Template
Valence Electrons And Ions Worksheets Answer Key
Valence Electrons And Ions Worksheets Answer Key
Valence Electrons Are The S And P Electrons In The Outermost Shell.
Follow The Instructions To Go Through The Simulation.
Web An Electron In The Highest Occupied Energy Level Of An Element's Atom.
Answer Key Ions Worksheet Element # Valence Electrons # Electrons.
Related Post:


![[EXCLUSIVE] Valence Electrons Chart Worksheet Answers](http://online.anyflip.com/tesy/ugkx/files/mobile/1.jpg)