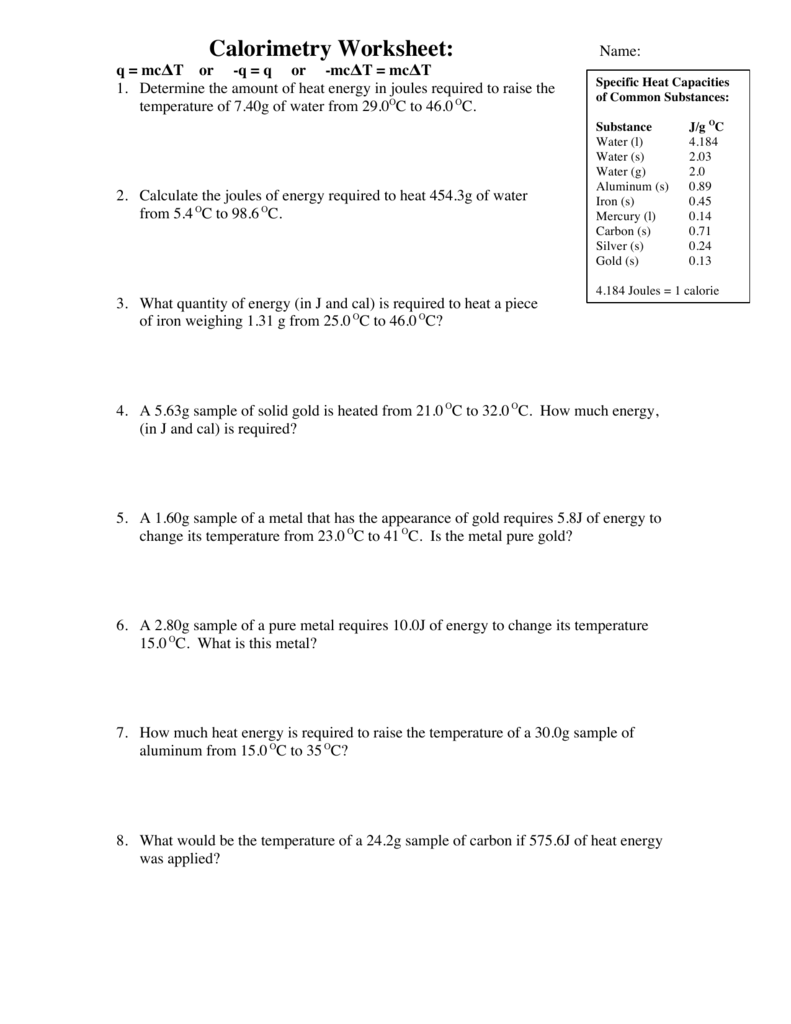
Calorimetry Worksheet Answers
Calorimetry Worksheet Answers - Web up to 24% cash back calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. It’s about understanding the process of problem. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it. Web specific heat and calorimetry (heat lost=heat gained) worksheet. Follow the instructions to go through the simulation. Choose an answer and hit 'next'. Web chemistry questions and answers. The final temperature is exactly halfway. The heat of the reaction is not directly observed, but. If the combustion of 0.175. Choose an answer and hit 'next'. Web up to 24% cash back worksheet #3 answers: For the electronic transition from n = 3 to n = 5 in the hydrogen atom. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Choose an answer and hit 'next'. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Web d50) practice calorimetry answers.pdf. Respond to the questions and prompts in the orange boxes. Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; In a constant volume apparatus like a calorimeter, the change in internal. If the combustion of 0.175. Web calorimetry heat absorbed or liberated by a. Web chemistry questions and answers; Web calorimetry worksheet answer key. The final temperature is exactly halfway. Temperature of water increases from 20.00 oc to 22.50 c.the Follow the instructions to go through the simulation. Temperature of water increases from 20.00 oc to 22.50 c.the If the combustion of 0.175. Choose an answer and hit 'next'. It’s about understanding the process of problem. Web chemistry questions and answers. The heat of the reaction is not directly observed, but. Web calorimetry problems worksheet answers march 24, 2023 by tamble it isn’t just about getting the right answers; Calorimetry is a technique for determining the enthalpy change of the reaction, as we discussed. The final temperature is closer to t_1 t 1 than to t_2 t 2. Web specific heat. If the combustion of 0.175. Calorimetry is a technique for determining the enthalpy change of the reaction, as we discussed. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it. A 2.50 g sample of zinc is heated, then placed in a calorimeter. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Web calorimetry problems worksheet answers march 24, 2023 by tamble it isn’t just about getting the right answers; Web chemistry questions and answers. Calorimetry is a technique for determining the enthalpy change of the reaction, as we discussed. What are the two. Web d50) practice calorimetry answers.pdf. Web up to 24% cash back calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; The final temperature is exactly halfway. Web up to 24% cash back. The final temperature is closer to t_1 t 1 than to t_2 t 2. Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water. Web an important idea in solving. The final temperature is closer to t_1 t 1 than to t_2 t 2. It’s about understanding the process of problem. Respond to the questions and prompts in the orange boxes. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water. Web up to 24% cash back calorimetry practice worksheet (section 17.2) 1.) compound a is burned in a bomb calorimeter that contains 2.50 liters of water. Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. Calorimetry is a technique for determining the enthalpy change of the reaction, as we discussed. How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? Web d) what is the heat capacity of the 5 g sample?5 g x 4 j/g * c = 22 j/ oc. The heat of the reaction is not directly observed, but. Web an important idea in solving calorimetry problems is that during a heat transfer between objects isolated from their surroundings, the heat gained by the colder. Web chemistry questions and answers. (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; The final temperature is exactly halfway. Temperature of water increases from 20.00 oc to 22.50 c.the Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Choose an answer and hit 'next'. Follow the instructions to go through the simulation. For the electronic transition from n = 3 to n = 5 in the hydrogen atom. The final temperature is closer to t_1 t 1 than to t_2 t 2. Calorimetry 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it. Web specific heat and calorimetry (heat lost=heat gained) worksheet. The heat of the reaction is not directly observed, but. Web calorimetry heat absorbed or liberated by a chemical reaction can be determined with a calorimeter. The final temperature is closer to t_1 t 1 than to t_2 t 2. Chapter 6 calorimetry worksheet key open the calorimetry lab activity (section 6.6, figure 6.11) to complete this worksheet. Web an important idea in solving calorimetry problems is that during a heat transfer between objects isolated from their surroundings, the heat gained by the colder. Calorimetry is a technique for determining the enthalpy change of the reaction, as we discussed. Web chemistry questions and answers; (heat capacity is the amount of energy to increase the temperature of an object by 1 oc; How much heat is required to raise the temperature of 67.0g of water from 25.7(c to 66.0(c? What are the two major ways in which the. Respond to the questions and prompts in the orange boxes. Web up to 24% cash back worksheet #3 answers: Choose an answer and hit 'next'. A 2.50 g sample of zinc is heated, then placed in a calorimeter containing 65.0 g of water.Calorimetry Worksheet Answer Key Kayra Excel
Calorimetry Worksheet Answer Key Kayra Excel
Calorimetry Worksheet Answer Key
calorimetry calculations worksheet
Calorimetry Worksheet Answer Key Kayra Excel
50 Calorimetry Worksheet Answer Key Chessmuseum Template Library
Calorimetry Worksheet Answer Key
Calorimetry Worksheet Answer Key
calorimetry practice worksheet
Calorimetry Worksheet Answers
In A Constant Volume Apparatus Like A Calorimeter, The Change In Internal.
The Final Temperature Is Exactly Halfway.
It’s About Understanding The Process Of Problem.
If The Combustion Of 0.175.
Related Post: