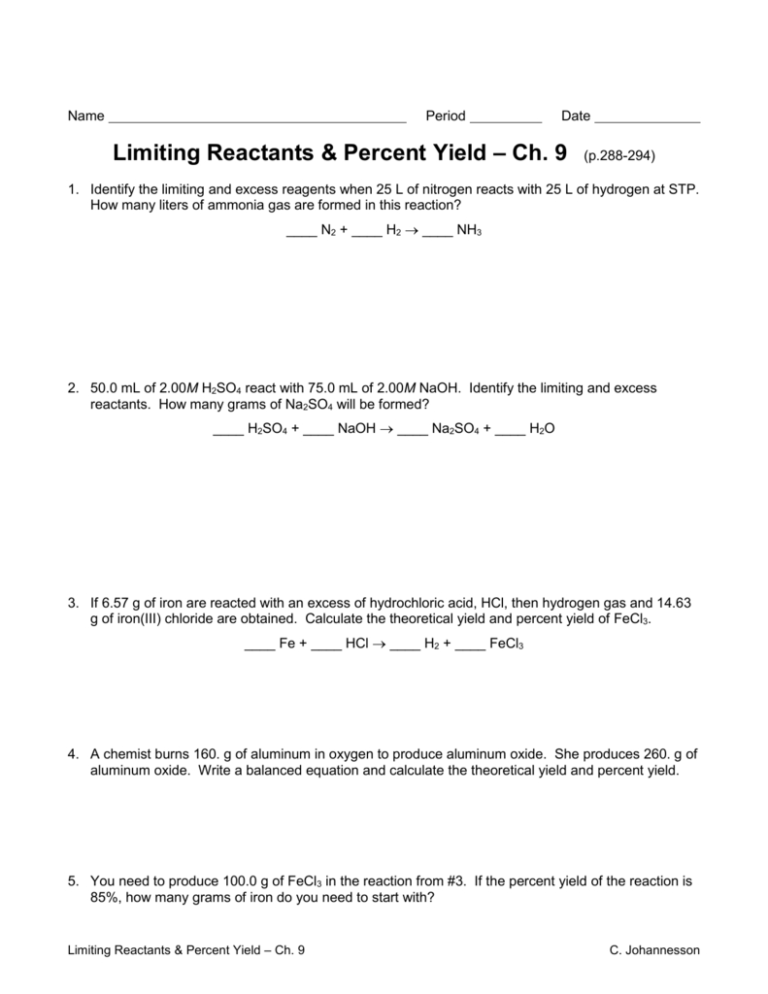
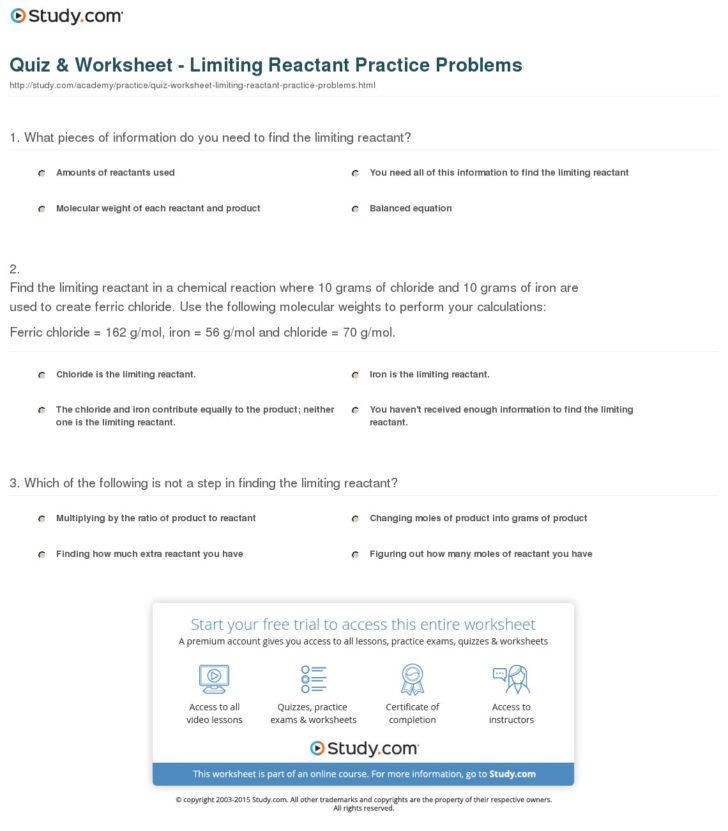
Limiting And Excess Reactants Worksheet Answers
Limiting And Excess Reactants Worksheet Answers - Calculate the limiting reagent in 2h 2 + o 2 → 2h 2 o. Web if 28 g of c3h8 and 45 g of o2 are reacted together, which one would be the limiting reactant?. Web a) which compound will be the limiting reagent? How many moles of oxygen can be produced? All of the questions on this worksheet involve the following reaction: Web what is the limiting reagent? Limiting and excess reactants other contents: 0 mol ko 2 x 3 mol o 2 = 0 mol o 2 4 mol. The limiting reagent would be o 2. Zn b) how many grams of zns will be formed? Worksheets are limiting reagent work, limiting reagents,. Mole add to my workbooks (0). 0.3803 mol = 37.1 g c) how many grams of the excess reactant will remain after the. Web limiting and excess reagents. Use the following equation to answer. Web a) which chemical is the limiting reactant? Can use either of the following to determine the limiting reactant. C) calculate the number of moles of the excess reagent remaining at the end. Web a complete, no prep worksheet that includes 4 problems requiring students to write and balance a chemical equation, then determine the limiting and excess reactants using.. If more is required, then b is the limiting. B) how many grams of no2 will be produced? Calculate the limiting reagent in 2h 2 + o 2 → 2h 2 o. The limiting reagent would be o 2. Worksheets are limiting reagent work, limiting reagents,. Can use either of the following to determine the limiting reactant. B) how many grams of no2 will be produced? How many moles of oxygen can be produced? Limiting and excess reactants other contents: Web what is the limiting reagent? Web limiting and excess reagents. Web if a reaction vessel contains 0 mol ko 2 and 0 mol h 2 o, what is the limiting reactant? Web a) which chemical is the limiting reactant? 0.3803 mol = 37.1 g c) how many grams of the excess reactant will remain after the. How many moles of oxygen can be produced? Web what is the limiting reactant? C) calculate the number of moles of the excess reagent remaining at the end. Determine how much one of the reactant needs of the. Web if a reaction vessel contains 0 mol ko 2 and 0 mol h 2 o, what is the limiting reactant? B) how many grams of no2 will be produced? Web a) which chemical is the limiting reactant? Web if 28 g of c3h8 and 45 g of o2 are reacted together, which one would be the limiting reactant?. Web limiting and excess reagents. How many moles of oxygen can be produced? C) calculate the number of moles of the excess reagent remaining at the end. Given 1 mol of hydrogen and 1. Web a complete, no prep worksheet that includes 4 problems requiring students to write and balance a chemical equation, then determine the limiting and excess reactants using. Can use either of the following to determine the limiting reactant. C) calculate the number of moles of the excess reagent remaining at the end. Calculate. Zn b) how many grams of zns will be formed? B) how many grams of no2 will be produced? Use the following equation to answer. Web a) which compound will be the limiting reagent? Web what is the limiting reagent? When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. How much excess is left over? Web a) which chemical is the limiting reactant? Web a complete, no prep worksheet that includes 4 problems requiring students to write and balance a chemical equation, then determine the limiting and excess reactants using. Web if a reaction vessel contains 0 mol. Can use either of the following to determine the limiting reactant. Mole add to my workbooks (0). B) how many grams of no2 will be produced? Web limiting and excess reagents. Web how do you know which of two reactants is the limiting one? Determine how much one of the reactant needs of the. 0 mol ko 2 x 3 mol o 2 = 0 mol o 2 4 mol. You compare the calculated amount of b to the actual amount available. Given 1 mol of hydrogen and 1. Use the following equation to answer. Web a) which chemical is the limiting reactant? C) calculate the number of moles of the excess reagent remaining at the end. Web what is the limiting reagent? If more is required, then b is the limiting. Web if a reaction vessel contains 0 mol ko 2 and 0 mol h 2 o, what is the limiting reactant? Limiting and excess reactants other contents: Web a complete, no prep worksheet that includes 4 problems requiring students to write and balance a chemical equation, then determine the limiting and excess reactants using. When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. 0.3803 mol = 37.1 g c) how many grams of the excess reactant will remain after the. Web what is the limiting reactant? 0 mol ko 2 x 3 mol o 2 = 0 mol o 2 4 mol. Web if 28 g of c3h8 and 45 g of o2 are reacted together, which one would be the limiting reactant?. Web a complete, no prep worksheet that includes 4 problems requiring students to write and balance a chemical equation, then determine the limiting and excess reactants using. Mole add to my workbooks (0). B) how many grams of no2 will be produced? Web what is the limiting reagent? You compare the calculated amount of b to the actual amount available. Limiting and excess reactants other contents: Web a) which chemical is the limiting reactant? Web what is the limiting reactant? When copper (ii) chloride reacts with sodium nitrate, copper (ii) nitrate. Web limiting and excess reagents. Calculate the limiting reagent in 2h 2 + o 2 → 2h 2 o. C) calculate the number of moles of the excess reagent remaining at the end. Zn b) how many grams of zns will be formed? Can use either of the following to determine the limiting reactant.Limiting Reactant Worksheet Answers
Limiting Reactant Worksheet Answers —
Limiting And Excess Reactants Worksheet —
Limiting Reactants Worksheet
Limiting Reactant and percent yield worksheets
Limiting Reagent Worksheet
42 limiting reactant worksheet answers Worksheet Resource
Limiting And Excess Reactants Worksheet —
Limiting Reagent Worksheet Answers —
Limiting And Excess Reactants Worksheet —
The Limiting Reagent Would Be O 2.
Given 1 Mol Of Hydrogen And 1.
Use The Following Equation To Answer.
Web How Do You Know Which Of Two Reactants Is The Limiting One?
Related Post: