Mixed Gas Laws Worksheet Answer Key
Mixed Gas Laws Worksheet Answer Key - Web in a mixture of gases, each gas behaves as if it alone occupied the entire volume. Worksheets are mixed gas laws work, mixed gas laws practice work name p, mixed gas laws work, gas laws work, 3 gas laws and key, , extra practice mixed gas. Lesson plans & lecture outlines. The gas laws and the ideal gas equation The worksheet includes word problems that covers boyle's law,. Web this worksheet combines all the gas laws and delivers undergraduate practice identifying the known and unknown variables for well while identifying the lawyer they must use to solve the word problem. The gas in a sealed can is at a pressure of 3.00 atm at 25(c. 2) if 5.0 moles of o 2 0and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the. If the volume is changed to 3.15 liters, what would öe the new pressure in attn? N = pv = (2.8 atm)(98 l) rt (0.0821 l.atm/mol.k)(292 k) = 11 moles of gas. 2) if 5.0 moles of o 2 0and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the. The worksheet includes word problems that covers boyle's law,. 2) if 5.0 moles of o. Dalton's law sates that the total pressure of a gas mixture is the. Web mixed gas laws practice. If the volume is changed to 3.15 liters, what would öe the new pressure in attn? 359 kpa = _____ atm 10 c = _____ k 6.2 atm = _____ kpa 10k = _____ c for the. G a s l a w s : 2) if 5.0 moles of o 2 0and 3.0 moles. 2) if 5.0 moles of o. A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(c. Web mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? Web the new gas volume be?. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. Therefore, we can assign a pressure to each gas in a mixture, called its partial pressure, pi. Ra13ph had a helium balloon with a volume of 4.88 liters at 150 kpa of pressure. _____. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. Web the new gas volume be? 2) if 5.0 moles of o. 5.36 liters of nitrogen gas are at. 359 kpa = _____ atm 10 c = _____ k 6.2 atm = _____ kpa. The gas laws and the ideal gas equation A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(c. Ra13ph had a helium balloon with a volume of 4.88 liters at 150 kpa of pressure. 359 kpa = _____ atm 10 c = _____ k 6.2 atm = _____. _____ show all work for all problems i. A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(c. Would be the volume at qornnk 6. Ra13ph had a helium balloon with a volume of 4.88 liters at 150 kpa of pressure. Web this worksheet combines all the gas. A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(c. 1.0 atm = 101.3 kpa = 760 mmhg and 0 c = 273 k change the following units: Lesson plans & lecture outlines. The gas in a sealed can is at a pressure of 3.00 atm at 25(c.. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. 359 kpa = _____ atm 10 c = _____ k 6.2 atm = _____ kpa 10k = _____ c for the. N = pv = (2.8 atm)(98 l) rt (0.0821 l.atm/mol.k)(292 k) = 11. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. Worksheets are mixed gas laws work, mixed gas laws practice work name p, mixed gas laws work, gas laws work, 3 gas laws and key, , extra practice mixed gas. Would be the volume. 2) if 5.0 moles of o. Therefore, we can assign a pressure to each gas in a mixture, called its partial pressure, pi. 1.0 atm = 101.3 kpa = 760 mmhg and 0 c = 273 k change the following units: G a s l a w s : Worksheets are mixed gas laws work, mixed gas laws practice work name p, mixed gas laws work, gas laws work, 3 gas laws and key, , extra practice mixed gas. Web mixed gas laws worksheet 1) how many moles of gas occupy 98 l at a pressure of 2.8 atmospheres and a temperature of 292 k? 2) if 5.0 moles of o 2 0and 3.0 moles of n 2 are placed in a 30.0 l tank at a temperature of 25 c, what will the pressure of the. A sample of hydrogen exerts a pressure of. N = pv = (2.8 atm)(98 l) rt (0.0821 l.atm/mol.k)(292 k) = 11 moles of gas. The gas laws and the ideal gas equation If the volume is changed to 3.15 liters, what would öe the new pressure in attn? Dalton's law sates that the total pressure of a gas mixture is the sum of. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. What would the gas pressure in the can be at 52(c? * gas law lessons pdf. Web in a mixture of gases, each gas behaves as if it alone occupied the entire volume. Web the new gas volume be? Lesson plans & lecture outlines. _____ show all work for all problems i. Web this worksheet combines all the gas laws and delivers undergraduate practice identifying the known and unknown variables for well while identifying the lawyer they must use to solve the word problem. Therefore, we can assign a pressure to each gas in a mixture, called its partial pressure, pi. 359 kpa = _____ atm 10 c = _____ k 6.2 atm = _____ kpa 10k = _____ c for the. If the volume is changed to 3.15 liters, what would öe the new pressure in attn? A warning on the can tells the user not to store the can in a place where the temperature will exceed 52(c. A sample of hydrogen exerts a pressure of. Ra13ph had a helium balloon with a volume of 4.88 liters at 150 kpa of pressure. 5.36 liters of nitrogen gas are at. The worksheet includes word problems that covers boyle's law,. Web mixed gas laws practice. Web the new gas volume be? The gas laws and the ideal gas equation * gas law lessons pdf. 1.0 atm = 101.3 kpa = 760 mmhg and 0 c = 273 k change the following units: The gas in a sealed can is at a pressure of 3.00 atm at 25(c. Web this unit explores the physical nature of gases, the laws governing the behavior of gases, and applications of gases from air bags to ozone depletion. N = pv = (2.8 atm)(98 l) rt (0.0821 l.atm/mol.k)(292 k) = 11 moles of gas.mixed gas laws worksheet solutions
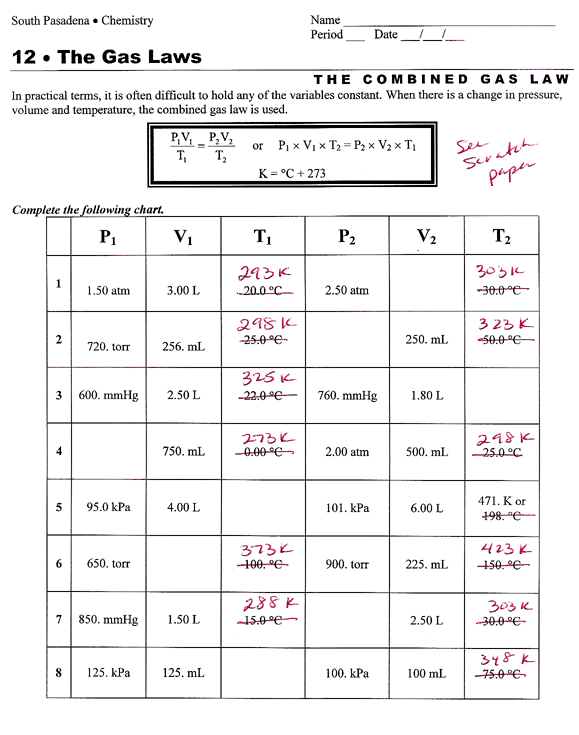
Combined Gas Law Worksheet Chart kulturaupice
Mixed Gas Laws Worksheet Answers —
Chemistry Mixed Gas Laws Worksheet Answer Key With Work Try this sheet
Combined Gas Law Worksheet Chart kulturaupice
Mixed Gas Law Worksheet Answer Key Uphandicrafts
️Gas Laws Worksheet Chemistry Answers Free Download Goodimg.co
16 Best Images of Mixed Gas Laws Worksheet Answers Mixed Gas Laws
Gas Law Review Worksheet Answers
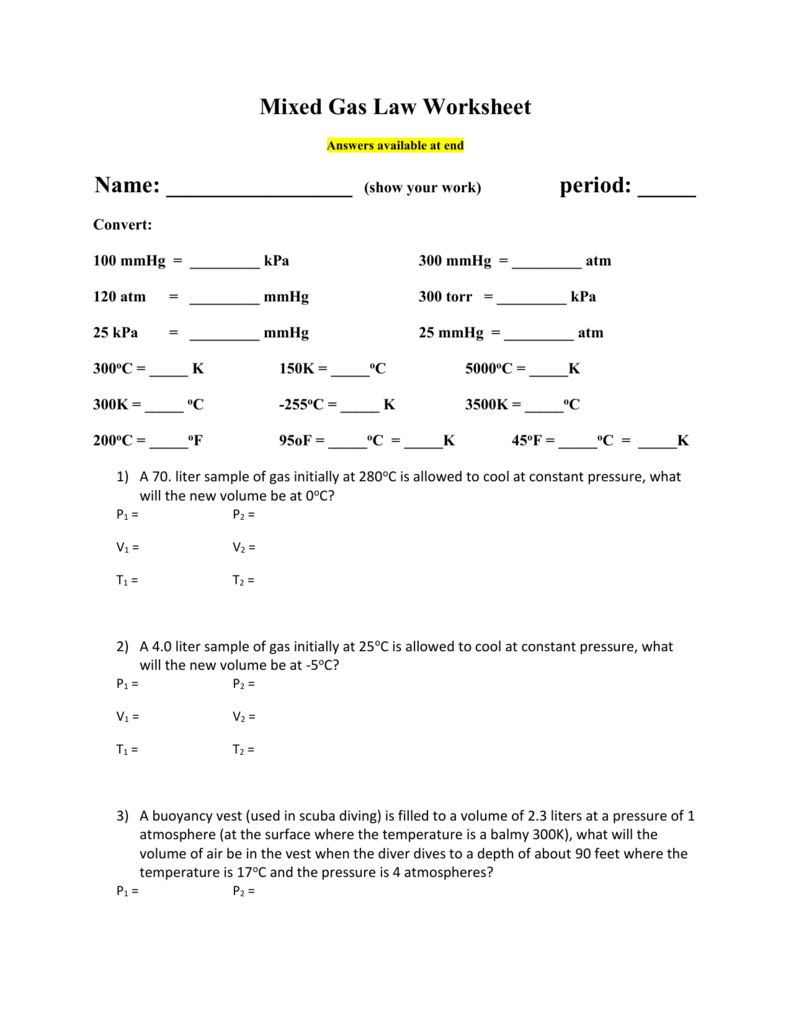
Mixed Gas Law Worksheet Answers available at end Name (show
2) If 5.0 Moles Of O 2 0And 3.0 Moles Of N 2 Are Placed In A 30.0 L Tank At A Temperature Of 25 C, What Will The Pressure Of The.
G A S L A W S :
Dalton's Law Sates That The Total Pressure Of A Gas Mixture Is The Sum Of.
What Would The Gas Pressure In The Can Be At 52(C?
Related Post: