Mole To Grams Grams To Moles Conversions Worksheet
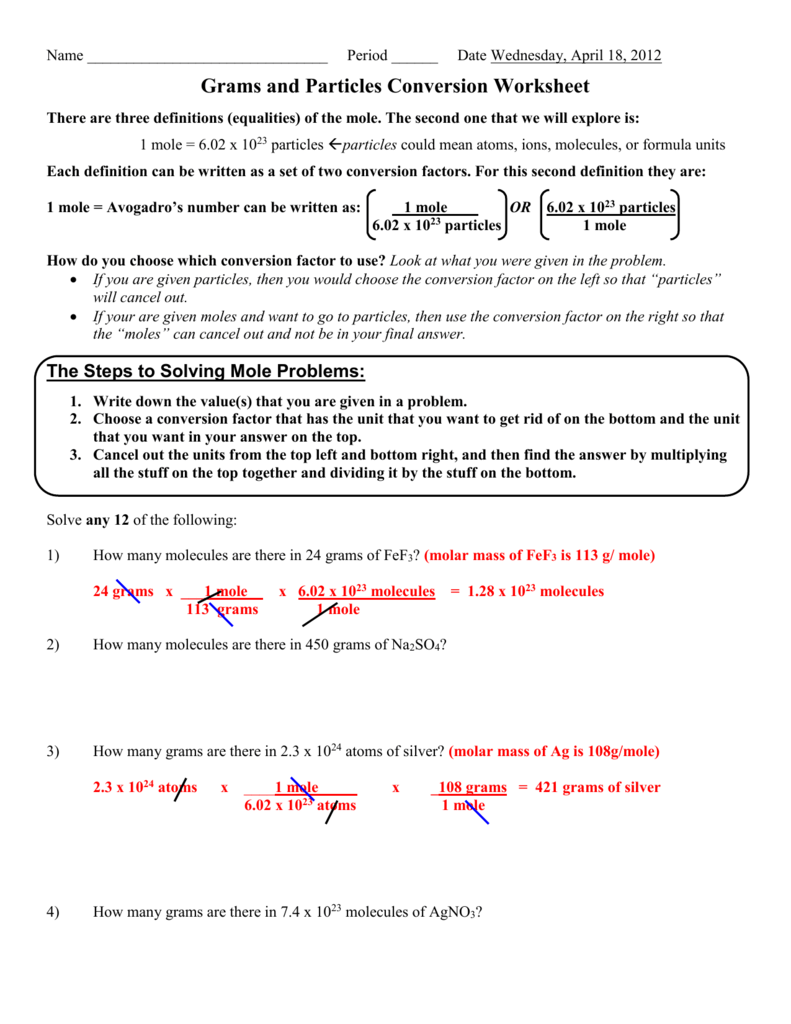
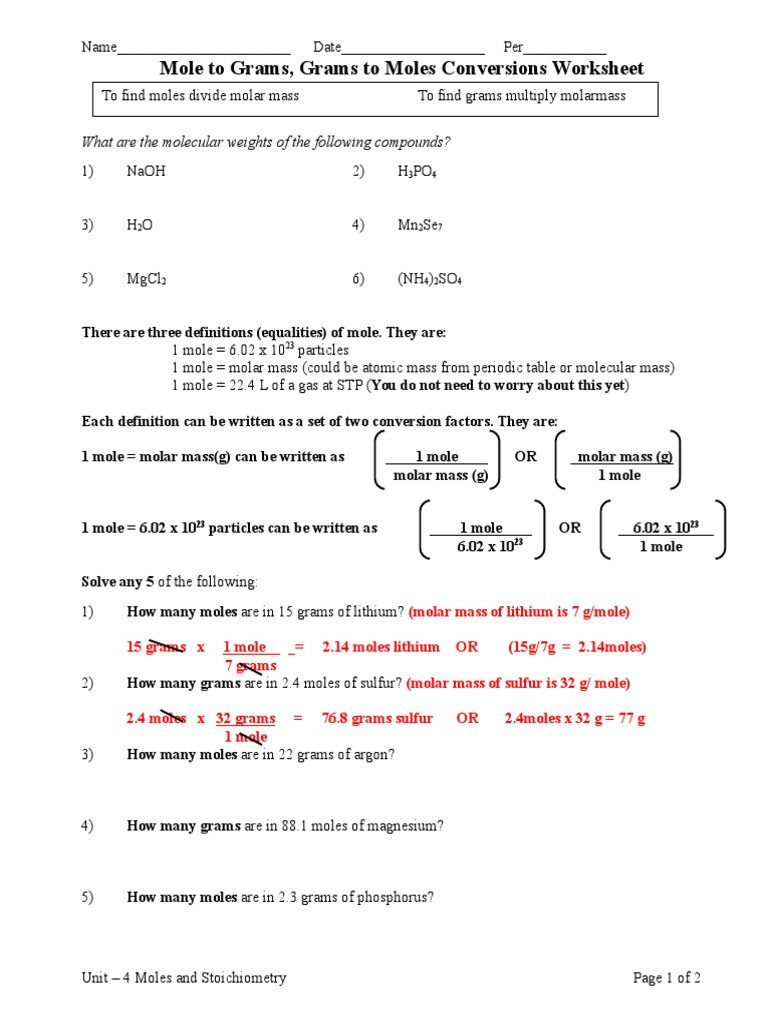
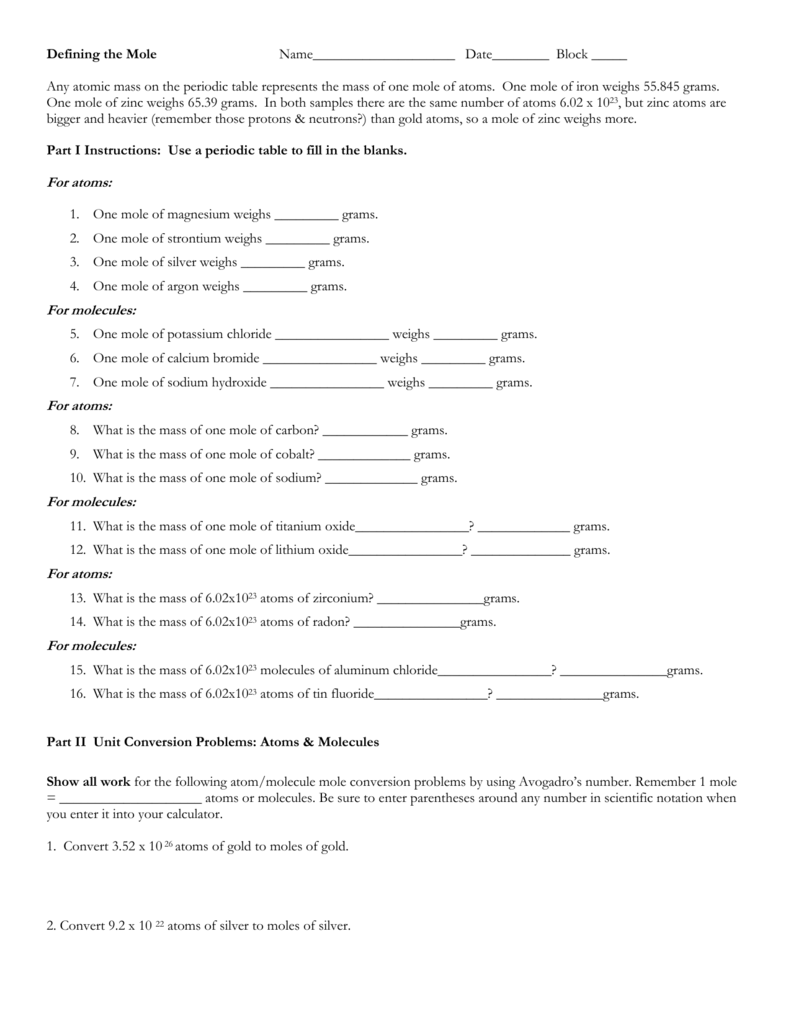
Mole To Grams Grams To Moles Conversions Worksheet - What is the mass of 5.00 moles of water(h 2 0)? How many moles in 28 grams of co2 ? 2 o = 2 x 16.00 g = 32.00 g. Determine the number of moles of a l 2 o 3 produced from 27.0 grams of a l. 2 h = 2 x( 1.01) = 2.02 #. Convert the following into moles: Calculation of molar mass and conversions between moles, mass and particles. 1 mol = molar mass in g. Moles, grams and particle conversions. Web step 1 is to determine and balance the equation. Given the following, name the compound and find the number of moles: Molar mass of a substance = mass of one mole of the substance. One mole of an element = the atomic mass of that element (from the. Web determine the number of mol of f e produced from 1.0 moles of f e 3 o 4. What is. 64.00 g/mol 28 g co2 =. Web step 1 is to determine and balance the equation. Determine the number of moles of a l 2 o 3 produced from 27.0 grams of a l. Worksheet #1 has students practice mole to mole ratios in chemical reactions. This assumes you are giving students word equations, or unbalanced equations. Some of the worksheets for this concept are mole to grams grams to moles conversions work, mole. 2 o = 2 x 16.00 g = 32.00 g. How many moles in 28 grams of co2 ? How many moles in 28 grams of co2 ? There are three mole equalities. Convert the following into moles: Find the number of moles of argon in 452 g of argon. 1 c = 1 x 12.01 g = 12.01 g. What is the mass of 5 moles of fe2o3 ? 64.00 g/mol 28 g co2 =. Web determine the number of mol of f e produced from 1.0 moles of f e 3 o 4. 2 h = 2 x( 1.01) = 2.02 #. There are three mole equalities. Step 2 is to convert the given information to moles. What is the mass of 5 moles of fe2o3 ? Molar mass of a substance = mass of one mole of the substance. Find the number of moles of argon in 452 g of argon. Determine the number of moles of a l 2 o 3 produced from 27.0 grams of a l. Some of the worksheets for this concept are mole to grams grams to moles conversions work, mole.. 2 o = 2 x 16.00 g = 32.00 g. Calculation of molar mass and conversions between moles, mass and particles. Moles, grams and particle conversions. Web step 1 is to determine and balance the equation. One mole of an element = the atomic mass of that element (from the. What is the mass of 5 moles of fe2o3 ? This assumes you are giving students word equations, or unbalanced equations. 64.00 g/mol 28 g co2 =. 2 o = 2 x 16.00 g = 32.00 g. 1 c = 1 x 12.01 g = 12.01 g. What is the mass of 5 moles of fe2o3 ? 2 o = 2 x 16.00 g = 32.00 g. Convert the following into moles: One mole of an element = the atomic mass of that element (from the. Worksheet #1 has students practice mole to mole ratios in chemical reactions. Web step 1 is to determine and balance the equation. Find the number of moles of argon in 452 g of argon. 2 o = 2 x 16.00 g = 32.00 g. How many moles in 28 grams of co2 ? Some of the worksheets for this concept are mole to grams grams to moles conversions work, mole. There are three mole equalities. Convert the following into moles: Web step 1 is to determine and balance the equation. 1 mol = 6.02 x 1023 particles. Given the following, name the compound and find the number of moles: 2 o = 2 x 16.00 g = 32.00 g. 1 c = 1 x 12.01 g = 12.01 g. This assumes you are giving students word equations, or unbalanced equations. How many moles in 28 grams of co2 ? Step 2 is to convert the given information to moles. Determine the number of moles of a l 2 o 3 produced from 27.0 grams of a l. Some of the worksheets for this concept are mole to grams grams to moles conversions work, mole. One mole of an element = the atomic mass of that element (from the. What is the mass of 5.00 moles of water(h 2 0)? Web gram to mole conversions: 64.00 g/mol 28 g co2 =. What is the mass of 5 moles of fe2o3 ? Web this work sheet allows students to find molar mass of four compounds and then take that knowledge to convert different measurement of grams to moles, moles to molecules,. Worksheet #1 has students practice mole to mole ratios in chemical reactions. Calculation of molar mass and conversions between moles, mass and particles. What is the mass of 5.00 moles of water(h 2 0)? 1 mol = 6.02 x 1023 particles. Find the number of moles of argon in 452 g of argon. How many moles in 28 grams of co2 ? Web step 1 is to determine and balance the equation. One mole of an element = the atomic mass of that element (from the. Molar mass of a substance = mass of one mole of the substance. There are three mole equalities. Calculation of molar mass and conversions between moles, mass and particles. 1) 30 grams of h3po4(phosphoric acid) 0.31 moles of h3po4. Convert the following into moles: 2 o = 2 x 16.00 g = 32.00 g. Web gram to mole conversions: Step 2 is to convert the given information to moles. How many moles in 28 grams of co2 ? 2 h = 2 x( 1.01) = 2.02 #.Moles Molecules And Grams Worksheet
Mole To Grams Grams To Moles Conversions Worksheet Answer Key — db
16 Best Images of Mole To Mole Worksheets Mole Molecules and Grams
Mole To Grams Grams To Moles Conversions Worksheet Answers —
10++ Mole Conversion Practice Problems Worksheet With Answers
Mole To Grams Grams To Moles Conversions Worksheet Answers Addition
Grams To Moles Worksheet. Worksheets. Free printable
Worksheet 3 Converting between grams, moles, and atoms
Mole Conversion Worksheet 1 Art Sense
Mole Conversions Worksheet
Some Of The Worksheets For This Concept Are Mole To Grams Grams To Moles Conversions Work, Mole.
1 C = 1 X 12.01 G = 12.01 G.
1 Mol = Molar Mass In G.
What Is The Mass Of 5 Moles Of Fe2O3 ?
Related Post: