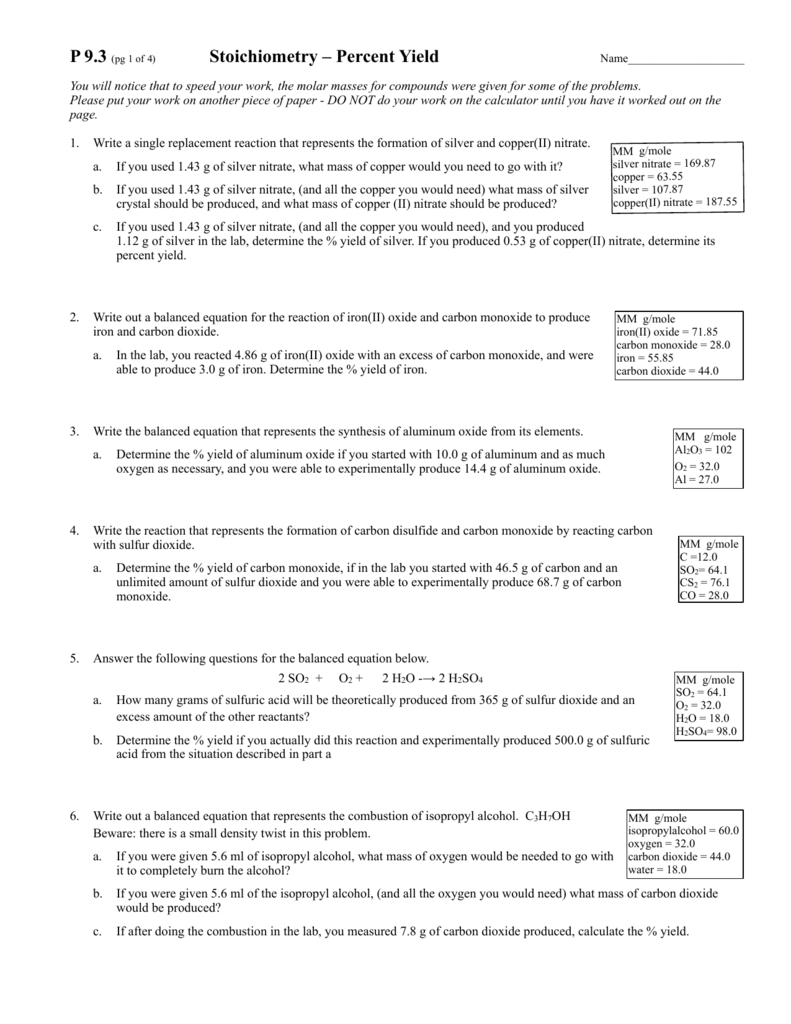
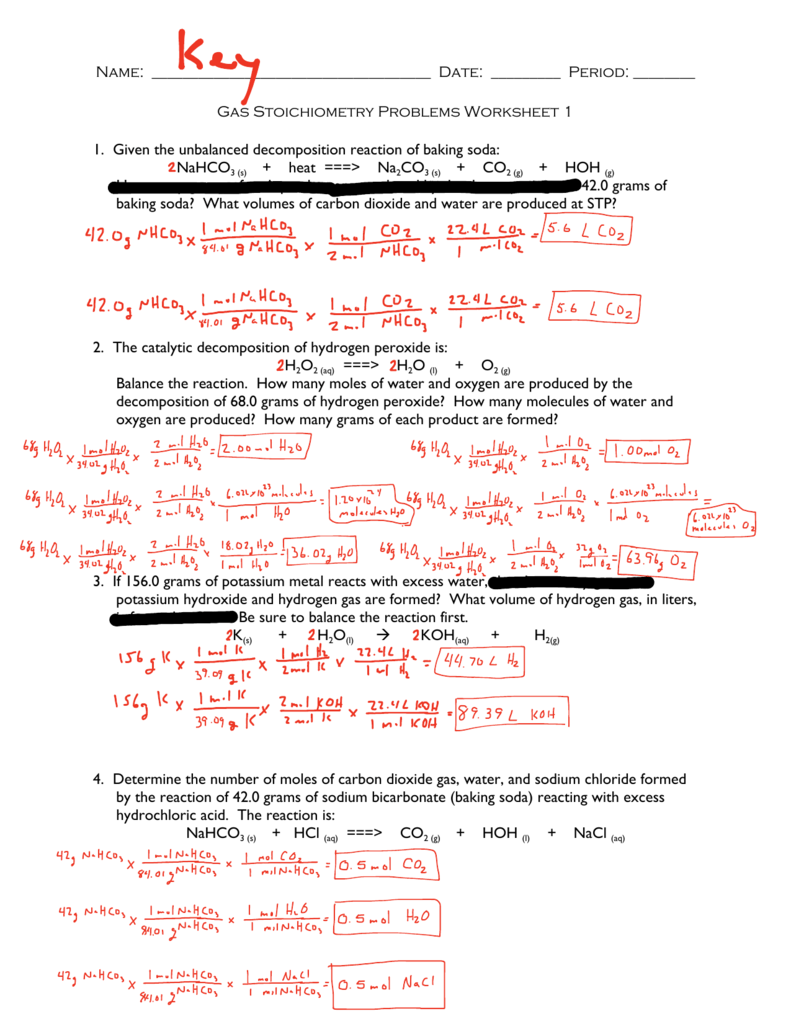Stoichiometry Worksheet 2 Percent Yield Answers
Stoichiometry Worksheet 2 Percent Yield Answers - The following diagram shows the. Determine the mass of water vapor you would expect to form (and the percent yield) in the reaction between 15.8 g of nh3 and excess oxygen to produce water. This is called the theoretical yield , the. Web percent yield calculations: The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Using theoretical and actual yields to determine whether the reaction was a success. Web up to 24% cash back 8. Percent yield for each of the problems below: Web stoichiometry worksheets and online activities. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass.. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Percent yield for each of the problems below: This printable was uploaded at march 18, 2023 by. This printable was uploaded at march 18, 2023 by tamble in answers. Web up to 24% cash back 8. Web stoichiometry worksheet 2 percent yield answers kayra excel is a free printable for you. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Web stoichiometry worksheets. Write the balanced chemical equation. Write the balanced chemical equation. Percent yield for each of the problems below: Web up to 24% cash back 8. Calculate the empirical formula of a. Write the balanced chemical equation b. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Web stoichiometry worksheet 2 percent yield answers kayra excel is a free printable for you. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and. Percent yield for each of the problems below: This printable was uploaded at march 18, 2023 by tamble in answers. Limiting reactant and percentage yield. In this lesson, we will learn. Web a series of free high school chemistry lessons. Web stoichiometry worksheets and online activities. The following diagram shows the. This is called the theoretical yield , the. This printable was uploaded at march 18, 2023 by tamble in answers. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Identify the given (with units) and what you want to find. Write the balanced chemical equation. Calculate the empirical formula of a. Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h. Write the balanced chemical equation b. Percent yield for each of the problems below: Web 2 +h 2 so 4 na 2 so 4 + mnso 4 + h 2 o + i 2 what is the percent yield of i 2 if the actual grams produced is 39.78 grams of i 2 from 62.55 grams of nai and excess. Using theoretical and actual yields to determine whether the reaction was a success. Web percent yield calculations: Percent yield for each of the problems below: Web modeling chemistry 1 u7 ws 2 v2.0name date pd stoichiometry worksheet 2: Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on. Web stoichiometry worksheets and online activities. Using theoretical and actual yields to determine whether the reaction was a success. Web up to 24% cash back 8. Write the balanced chemical equation. Free interactive exercises to practice online or download as pdf to print. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. The following diagram shows the. This is called the theoretical yield , the. Web a series of free high school chemistry lessons. Limiting reactant and percentage yield. This printable was uploaded at march 18, 2023 by tamble in answers. In this lesson, we will learn. Write the balanced chemical equation b. Web stoichiometry worksheet 2 percent yield answers kayra excel is a free printable for you. 0/0 yield = actual yield x 10th theoretical yeild theoretical yield = answer to your stoich problem. Write the balanced chemical equation. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Percent yield for each of the problems below: Identify the given (with units) and what you want to find. Web percent yield calculations: Percent yield for each of the problems below: Web live worksheets > english > chemistry > stoichiometry > percentage yield exercises. Web to compute the percent yield, it is first necessary to determine how much of the product should be formed based on stoichiometry. Percent yield for each of the problems below: Percent yield for each of the problems below: Write the balanced chemical equation b. The amount of product that may be produced by a reaction under specified conditions, as calculated per the stoichiometry of an appropriate balanced chemical. This is called the theoretical yield , the. Free interactive exercises to practice online or download as pdf to print. Web a series of free high school chemistry lessons. Web convert between numbers of atoms, moles, and mass of sample by using avogadro’s number and the appropriate molar mass. Web up to 24% cash back 8. Determine the mass of water vapor you would expect to form (and the percent yield) in the reaction between 15.8 g of nh3 and excess oxygen to produce water. Calculate the empirical formula of a. Web stoichiometry worksheet 2 percent yield answers kayra excel is a free printable for you. Using theoretical and actual yields to determine whether the reaction was a success.Limiting Reactant And Percent Yield Worksheet Answers Worksheet Now
Stoichiometry Worksheet 2 Percent Yield Answers Kayra Excel
14 Stoichiometry Worksheet 2 Answer Key /
Stoichiometry Percent Yield
Stoichiometry Worksheet Answer Key
Stoichiometry Worksheet 2 Percent Yield Answers Kayra Excel
Stoichiometry, Limiting Reagent, Percent Yield YouTube
Stoichiometry Percent Yield, Practice Problem 2 YouTube
Limiting Reagents and Percentage Yield Worksheet answers.doc Zinc
Stoichiometry Worksheet 2 Percent Yield Answers Tomas Blog
Web 2 +H 2 So 4 Na 2 So 4 + Mnso 4 + H 2 O + I 2 What Is The Percent Yield Of I 2 If The Actual Grams Produced Is 39.78 Grams Of I 2 From 62.55 Grams Of Nai And Excess Of All The.
Web Stoichiometry Worksheets And Online Activities.
Write The Balanced Chemical Equation.
Limiting Reactant And Percentage Yield.
Related Post: